| Identification | More | [Name]
Hydrochlorothiazide | [CAS]
58-93-5 | [Synonyms]
3,4-dihydro-6-chloro-7-sulfamoyl-1,2,4-benzothiadiazine-1,1-dioxide
3,4-dihydrochlorothiazide
6-CHLORO-1,1-DIOXO-1,2,3,4-TETRAHYDRO-1LAMBDA6,2,4-BENZOTHIADIAZINE-7-SULFONAMIDE
6-CHLORO-3,4-DIHYDRO-2H-1,2,4-BENZOTHIADIAZINE-7-SULFONAMIDE 1,1-DIOXIDE
6-chloro-3,4-dihydro-7-sulfamoyl-2h-1,2,4-benzothiadiazine-1,1-dioxide
6-CHLORO-7-SULFAMYL-3,4-DIHYDRO-1,2,4-BENZOTHIADIAZINE 1,1-DIOXIDE
HYDROCHLOROTHIAZIDE
HYDROCHLORTHIAZIDE
LABOTEST-BB LT00134623
2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide
2h-1,2,4-benzothiadiazine-7-sulfonamide,6-chloro-3,4-dihydro-,1,1-dioxide
3,4-Dihydro-6-chloro-7-sulfamyl-1,2,4-benzothiadiazine-1,1-dioxide
6-chloro-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide
6-Chloro-3,4-dihydro-7-sulphamoyl-1,2,4-ben-zoth-iadi-azine1,1-dioxcide
6-chloro-7-sulfamoyl-3,4-dihydro-2h-1,2,4-benzothiadiazine
6-Chloro-7-sulfamoyl-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide
6-chloro-7-sulfamoyl-3,4-dihydro-2h-1,2,4-benzothiadiazine-1,1-dioxide
6-chloro-7-sulfamoyl-3,4-dihydro-2h-1,2,4-benzothiadiazine1,1-dioxide
Acuretic
Aldactazide | [EINECS(EC#)]
200-403-3 | [Molecular Formula]
C7H8ClN3O4S2 | [MDL Number]
MFCD00051765 | [Molecular Weight]
297.74 | [MOL File]
58-93-5.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
273 °C | [Boiling point ]
577.0±60.0 °C(Predicted) | [density ]
1.6761 (rough estimate) | [refractive index ]
1.6100 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
Very slightly soluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides | [form ]
solid | [pka]
7.9, 9.2(at 25℃) | [color ]
White to Off-White | [Odor]
wh. or pract. wh. cryst. powd., odorless | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [Water Solubility ]
722mg/L(25 ºC) | [Usage]
A carbonic anhydrase inhibitor | [λmax]
318nm(H2O)(lit.) | [Merck ]
14,4781 | [BRN ]
625101 | [BCS Class]
3,4 | [Major Application]
pharmaceutical (small molecule) | [InChI]
1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13) | [InChIKey]
JZUFKLXOESDKRF-UHFFFAOYSA-N | [SMILES]
NS(=O)(=O)c1cc2c(NCNS2(=O)=O)cc1Cl | [LogP]
-0.070 | [CAS DataBase Reference]
58-93-5(CAS DataBase Reference) | [IARC]
2B (Vol. 50, 108) 2016 | [NIST Chemistry Reference]
6-Chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide(58-93-5) | [EPA Substance Registry System]
58-93-5(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,T,F | [Risk Statements ]
R22:Harmful if swallowed.
R42/43:May cause sensitization by inhalation and skin contact .
R36/38:Irritating to eyes and skin .
R23/25:Toxic by inhalation and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24:Avoid contact with skin .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S33:Take precautionary measures against static discharges .
S16:Keep away from sources of ignition-No smoking .
S7:Keep container tightly closed .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S27:Take off immediately all contaminated clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 1230 3/PG 2 | [WGK Germany ]
2 | [RTECS ]
DK9100000 | [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29350090 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Carc. 2 | [Safety Profile]
Poison bp
intraperitoneal and intravenous routes.
Moderately toxic by ingestion and
subcutaneous routes. Human systemic
effects by ingestion: sodum level changes,
chlorine level changes, acute pulmonary
edema, nausea or vomiting. Experimental
reproductive effects. Questionable
carcinogen with experimental tumorigenic
data. Mutation data reported. A duetic.
When heated to decomposition it emits very
toxic fumes of SOx, Cl-, and NOx. | [Hazardous Substances Data]
58-93-5(Hazardous Substances Data) | [Toxicity]
LD50 in mice (mg/kg): 590 i.v.; >8000 orally (Piala) |
| Hazard Information | Back Directory | [General Description]
It is a white, crystalline, and odorless powder with a slightly bitter taste. It is insoluble in water and chloroform, soluble in acetone and sodium hydroxide solution, slightly soluble in ethanol, but is readily hydrolyzed
| [Reactivity Profile]
Strong reducing agents will produce toxic gases ammonia and hydrogen sulfide. | [Air & Water Reactions]
Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available but HYDROCHLOROTHIAZIDE is probably combustible. | [Originator]
Hydrodiuril,MSD,US,1959 | [Definition]
ChEBI: Hydrochlorothiazide is a benzothiadiazine that is 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide substituted by a chloro group at position 6 and a sulfonamide at 7. It is diuretic used for the treatment of hypertension and congestive heart failure. It has a role as a xenobiotic, an environmental contaminant, a diuretic and an antihypertensive agent. It is a benzothiadiazine, a sulfonamide and an organochlorine compound. | [Manufacturing Process]
As described in US Patent 3,163,645, a mixture of 2.9 grams of 5-chloro-2,4-
disulfamyl aniline in 15 ml of anhydrous diethyleneglycol dimethyl ether, 0.5
ml of an ethyl acetate solution containing 109.5 grams of hydrogen chloride
per 1,000 ml and 0.33 grams (0.011 mol) of paraformaldehyde is heated to
80° to 90°C and maintained at that temperature for 1 hour. The resulting
mixture is cooled to room temperature and concentrated to one-third of its volume under reduced pressure, diluted with water, then allowed to
crystallize. The product is filtered off and recrystallized from water, to yield
the desired 6-chloro-7-sulfamyl-3,4-dihydro-2H-[1,2,4]-benzothiadiazine-1,1-
dioxide, MP 266° to 268°C, yield 1.4 grams. By replacing paraformaldehyde
by 0.84 gram of 1,1 -dimethoxymethane and proceeding as above, the same
compound is obtained.
As described in US Patent 3,025,292, the desired product may be made by
hydrogenation of chlorothiazide. Three grams of 6-chloro-7-sulfamyl-1,2,4-
benzothiadiazine-1,1-dioxide (chlorothiazide) is suspended in 100 ml of
methanol. Then 1.0 gram of a 5% ruthenium on charcoal catalyst is added,
and the mixture is reduced at room temperature and at an initial hydrogen
pressure of 39 psig. The theoretical amount of hydrogen to form the 3,4-
dihydro derivative is absorbed after a period of about 10 hours.
The reduction mixture then is heated to boiling and filtered hot to remove the
catalyst. The catalyst is washed with a little methanol and the combined
filtrate is concentrated to a volume of about 25 ml by evaporation on a steam
bath. Upon cooling to room temperature, white crystals separate which are
filtered, washed with water, and dried in vacuo at room temperature over
phosphorus pentoxide overnight. The weight of 6-chloro-7-sulfamyl 3,4-
dihydro-1,2,4-benzothiadiazine-1,1-dioxide obtained is 1.26 grams; MP 268.5°
to 270°C. Dilution of the above filtrate with water to a volume of about 125
ml gives a second crop of product having the same melting point and
weighing 1.22 grams, giving a combined yield of 83%. When the product is
mixed with an authentic sample of 6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-
benzothiadiazine-1,1-dioxide, prepared by another method, the melting point
is not depressed. | [Brand name]
Esidrix (Novartis); Hydro-D (Halsey); Hydrodiuril
(Merck); Microzide (Watson); Oretic (Abbott); Zide
(Solvay Pharmaceuticals). | [Therapeutic Function]
Diuretic | [Biological Activity]
Carbonic anhydrase inhibitor | [Synthesis]
Hydrochlorothiazide, 1,1-dioxide 6-chloro-3,4-dihydro-2H-1,2,4-
benzothiadiazin-7-sulfonamide (21.3.4), is synthesized either by cyclization of 4,6-sulfonamido-
3-chloroaniline (21.3.2) using paraformaldehyde, during which simultaneous
reduction of the double bond occurs at position C3¨CC4, or the drug is synthesized by reduction
of the same double bond in chlorothiazide (21.3.3) by formaldehyde. This small change
in structure increases activity of the drug in comparison with chlorothiazide, and increases
its absorbability when used orally.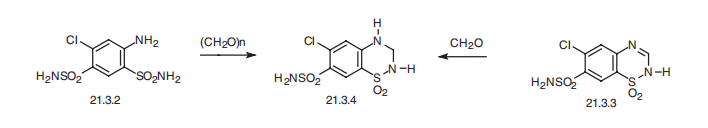
| [Veterinary Drugs and Treatments]
In veterinary medicine, furosemide has largely supplanted the use
of thiazides as a general diuretic (edema treatment). Thiazides are
still used for the treatment of systemic hypertension,
nephrogenic
diabetes insipidus, and to help prevent the recurrence of calcium
oxalate uroliths in dogs. | [References]
[1] Patent: WO2009/150497, 2009, A1. Location in patent: Page/Page column 9
[2] Pharmazie, 1987, vol. 42, # 3, p. 162 - 164
[3] Journal of the American Chemical Society, 1960, vol. 82, p. 1161 - 1166
[4] Journal of Organic Chemistry, 1960, vol. 25, p. 970 - 981
[5] Journal of the American Chemical Society, 1960, vol. 82, p. 1132 - 1135 |
| Questions And Answer | Back Directory | [description]
Hydrochlorothiazide is a kind of thiazide diuretic drugs with moderate diuretic effect. It exert is diuretic effect through acting on the medullary ascending limb cortical segment, inhibiting the active re-absorption of Cl-and passive absorption of Na+ at this site. In addition, the product also has a hypotensive effect and anti-diuretic effect. It is suitable for treating a variety of edema, particularly for cardiogenic edema. It can also be used as antihypertensive agents, for treatment light, medium hypertension. It also has certain efficacy in treating diabetes insipidus, and idiopathic hypercalciuria. Commonly used thiazide diuretics include hydrochlorothiazide, chlorthalidone, indapamide and the like.
| [Chemical Properties]
It is a white crystalline powder and is odorless with slightly bitter taste. It is insoluble in chloroform and water but soluble in acetone, slightly soluble in ethanol, soluble in sodium hydroxide solution but being susceptible to hydrolysis.

Figure 1 is the structure formula of hydrochlorothiazide
| [mechanism of action]
1. Natriuretic effect: This product mainly inhibits the re-absorption of Na+, Cl-of distal tubule anterior part and proximal tubules (mild effect), and increasing urinary sodium, potassium, chlorine, phosphorus and magnesium ion excretion, and reducing urinary calcium excretion.
2. Antihypertensive effect: it has a moderate and precise antihypertensive effect and can reduce both the orthostatic, supine systolic and diastolic pressure and also enhance the hypotensive effect of other antihypertensive drugs.
3. Anti-diuretic effect: This product can reduce the amount of urine in nephrogenic diabetes insipidus, sometimes by 50% with the specific mechanism of action remaining unknown.
| [Pharmacokinetics]
It has rapid but incomplete oral absorption with heaving meal being able to increase the absorbed amount, which may be related with the prolonged residence time of drug in the small intestine. This product can partially bind to the plasma protein with the other part getting into the red blood cells. It takes effect at 2h after oral administration with the peak reaching in 4h. It duration time is 6~12h. It is mainly secreted in the prototype through the urinary excretion with the half-life (t1/2) being 15h and the t1/2 being extended in renal dysfunction.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Indications]
1. Edema disease: including congestive heart failure, cirrhosis, nephrotic syndrome, acute and chronic nephritis edema, chronic renal failure early, adrenocorticotropic hormone and estrogen therapy caused retention of sodium and water.
2. High blood pressure: it can be used alone or in combination with other antihypertensive drugs. It is mainly used for the treatment of essential hypertension.
3. Central or nephrogenic diabetes insipidus.
4. Kidney stone disease: it is mainly used for the prevention of calcium stone formation.
| [Dosage]
Oral ordinary tablet: the efficacy onset of oral administration is in 2h with plasma concentration reaching peak at 4 hour and the effect lasting for 6~12h.
Adults: (1) for the treatment of edema disease, a 25~50mg, qd or bid, or qod; or take drug 3~5d weekly with a interval of 3~4d. (2) For the treatment of hypertension with the dose of 25 to 100 mg per day and divided into 1-2 times for administration. It needs to be used in combination with other antihypertensive drugs and should be subject to dose adjustment according to the actual antihypertensive effect. The dose is usually reduced to 25 to 50 mg per day within one week. (3) for the treatment of diabetes insipidus, take 25mg once tid, or 50 mg once, qd.
Children: treatment of edema disease, take 1~2mg/kg per day and divided 1-2 times for administration. Adjust the dose according to the actual efficacy.
| [Side effects]
Most adverse events are related to the dose and duration.
1. Water, electrolyte imbalance caused adverse reactions are the more common: hypokalemia is prone to occur and is related to the potassium excretion effect of thiazide diuretics. Long-term potassium deficiency can damage tubular with serious loss of potassium being able to cause vacuolar changes in the renal tubular epithelial and severe tachyarrhythmias and other ectopic rhythm. Thiazide class, especially hydrochlorothiazide can often significantly increases the excretion of chloride, causing low chlorine alkalosis or low chlorine, potassium alkalosis. In addition, hyponatremia is also not rare, causing central nervous system symptoms and aggravate kidney damage. Dehydration causes blood volume and renal blood flow reduction and can also cause reduced glomerular filtration rate. The common clinical manifestations of water, electrolyte imbalance include dry mouth, thirst, muscle cramps, nausea, vomiting and extreme fatigue, weakness and so on.
2. Hyperglycemia: It can make impaired glucose tolerance, elevated blood sugar, may be associated with inhibition of insulin release.
3. Hyperuricemia: it can interfere with renal tubular excretion of uric acid with a few being able to cause gout attacks. Usually it doesn’t cause joint pain, so hyperuricemia is easily overlooked.
4. Allergy: such as rash, urticaria; relatively rare.
5. Leukopenia or deficiency, and thrombocytopenic purpura, also rare.
6. Rare cholecystitis, pancreatitis, sexual dysfunction, light sensitivity, and color vision disorders.
| [Drug Interaction]
1. Adrenocorticotropic hormone, corticotropin, estrogens, amphotericin B (intravenous administration), can reduce the diuretic effect of this product and increase the chance of electrolyte imbalance, particularly hypokalemia.
2. Non-steroidal anti-inflammatory analgesic drugs, especially indomethacin, can reduce the diuretic effect of this product which is related with the former’s inhibition of prostaglandin synthesis.
3. Upon combination with the sympathomimetic amine drugs, its diuretic effect weakened.
4. Cholestyramine (cholestyramine) can reduce the gastrointestinal absorption of this product. Therefore, it should be taken orally at 1 h before or 4 h after taking the former drug.
5. In combination with dopamine, the diuretic effect strengthened.
6. In combination with antihypertensive drugs, both diuretic and antihypertensive effect were enhanced.
7. In combination with anti-gout drug, the dosage of the later one should be adjusted.
8. It can weaken the anticoagulant, mainly due to the decreased elevated levels of blood plasma volume after diuretic, elevated levels of blood clotting factors, together with the diuretic effect improving blood supplement in the liver, and increased synthesis of clotting factors.
9. It can reduce the role of hypoglycemic agents.
10. Upon being used combination with digitalis drugs or amiodarone, you should beware of adverse reactions caused by hypokalemia.
11. Upon being combination with lithium preparations, the product can reduce the renal clearance of lithium and increase renal toxicity of lithium.
12. The effect of methenamine is affected by this product with its conversion to formaldehyde being inhibited, decreasing the efficacy.
13. Enhance the non-depolarizing muscle relaxant effect which is related to a decline in serum potassium.
14. Upon being used in combination with sodium bicarbonate, the chances of low chlorine alkalosis increases.
| [Uses]
The product is a typical representative in the thiazide diuretic drug with its pharmaceutical sales in United States ranking first in 1985. Owing to its easy administration, moderate action as well as being suitable for all kinds of edema, it is most often clinically applied. Owing to adverse reactions like potassium secretion, we should pay attention to supplement potassium salt during the application. Intravenous injection-mice-LD50: 590mg/kg, oral LD50: greater than 8000mg/kg.
Diuretics
It mainly inhibits the re-absorption on Na+ and Cl-by the proximal end of the distal convoluted tubule so that renal excretion of sodium chloride is increased to produce a diuretic effect. It is a kind of diuretic drugs of moderate effect. This product has antihypertensive effect with the effect being enhanced when being use in combination with Lee medicine paste, antihypertensive shen paste or apocynum venetum and other traditional Chinese medicine. This product also has anti-diuretic effect and can be used for the treatment of diabetes insipidus
| [Production method]
It is produced by: m-Chlorosulfonated goes through chlorosulfonation to give 5-chloro-2,4-chloro-anilide, and further reacted with ammonia to form 5-chloro-2,4-sulfamoyl aniline which finally reacts with formaldehyde to obtain the final product.
|
|
|