| Identification | Back Directory | [Name]
(R)-4-Propyldihydrofuran-2(3H)-one | [CAS]
63095-51-2 | [Synonyms]
BR150
Brisetan
Boisacetan
Buvacitan impurity 11
Brivaracetam Impurity 13
Brivaracetam Impurity 40
(4R)-4-propyloxolan-2-one
Brivaracetam Intermediate
brivaracetam intermediate 1
(R)-4-propyl-dihydro-furan-2-one
(R)-4-propyI-dihydro-furan-2-one
(R)-4-propyldihydrofuran-2(3H)-one
(4R)-4-propyltetrahydrofuran-2-one
(R)-Dihydro-4-propylfuran-2(3H)-one
(4R)-4-Propyldihydrofuran-2(3H)-one
(R)-Dihydro-4-propyl-2(3H)-furanone
2(3H)-Furanone,dihydro-4-propyl-,(R)-
(R)-4-propylidihydrofuran-2(3H)-4-one
(+)-(R)-4-propyl-4,5-dihydrofuran-2(3H)-one
(+)-(R)-4-propyl-4,5-dihydroturan-2(3H)-one
(+)-(R)-4-propyl-4,5- dihydrofuran-2(3H)-one
2(3H)-Furanone, dihydro-4-propyl-, (R)- (9CI)
(R)-4-propyl-4,5-dihydrofuran-2(3H)-one(BR50)
(R)-4-Propyldihydrofuran-2(3H)-one(For export only)
(R)-4-Propyldihydrofuran-2(3H)-one(Briveracetam KSM)
(R)-4-Propyldihydrofuran-2(3H)-One/ Intermediate Of Brivaracetam | [EINECS(EC#)]
13-650-7 | [Molecular Formula]
C7H12O2 | [MDL Number]
MFCD29905061 | [MOL File]
63095-51-2.mol | [Molecular Weight]
128.17 |
| Chemical Properties | Back Directory | [Boiling point ]
226.3±8.0 °C(Predicted) | [density ]
0.983±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) | [form ]
Oil | [color ]
Colourless |
| Hazard Information | Back Directory | [Description]
(R)-4-Propyldihydrofuran-2(3H)-one is the key intermediate in the diastereoselective synthesis of
Brivaracetam, an active ingredient with antiepileptic properties
belonging to the racetam family. | [Chemical Properties]
(R)-4-Propyldihydrofuran-2(3H)-one is a yellow oily liquid, slightly
soluble in chloroform, methanol and ethanol. It needs to be stored at
2-8°C.
| [Uses]
(R)-Dihydro-4-propyl-2(3H)-furanone, is building block used in various chemical synthesis. | [Synthesis]
Add a solution of H5IO6 (24.429 g, 107.17 mmol) in water (43 mL) to a solution of (R)-2-benzylpentyl acetate (1.180 g) in CCl4 (27 mL) and CH3CN (27 mL) at 0 °C. Add RuCl3·nH2O
(0.222 g, 1.07 mmol) slowly to the mixture at 0 °C. Stir the resultant
mixture vigorously for 20 hours at room temperature. Quench the reaction
with Et2O (50 mL) at 0 °C. Stir the mixture for 30 minutes. Extract the
mixture with Et2O (3 × 50 mL).
Wash the combined organic layers with brine (30 mL). Dry the combined organic layers over anhydrous Na2SO4.
Filter the combined organic layers. Remove the solvent. Dissolve the
oil in aqueous NaOH (1 mol L-1, 40 mL). Stir the resulting solution
overnight at room temperature. Wash the solution with Et2O
(30 mL). Acidify the solution with aqueous HCl (6 mol L-1, 15 mL) at 0
°C. Stir the mixture overnight at room temperature. Saturate the aqueous
layer with NaCl. Extract the mixture with Et2O (5 × 40 mL). Wash the combined organic layers with water, saturated aqueous Na2S2O3 (30 mL) and brine (30 mL).
Dry the combined organic layers over anhydrous Na2SO4.
Filter the combined organic layers. Remove the solvent. Purify the
residue by column chromatography on silica gel (hexane/acetone = 3/1)
and distillation (162-209 °C/36 mmHg).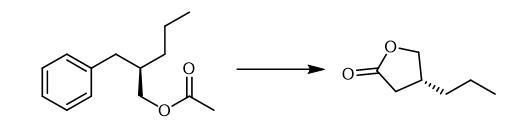 |
|
|