| Identification | More | [Name]
Unii-33Y9anm545 | [CAS]
635702-64-6 | [Synonyms]
786034
ArMala
Pazopanib HCI
Pazopanib HCl
Unii-33Y9anm545
Pazopanib hydrochloride
Pazopanib HCl (GW786034 HCl)
Pazopanib Hydrochloride (GW786034)
5-(4-((2,3-diMethyl-2H-indazol-6-yl)(Methyl)aMino)pyriMidin-2-ylaMino)-2-MethylbenzenesulfonaMide hydrochloride
BenzenesulfonaMide, 5-[[4-[(2,3-diMethyl-2H-indazol-6-yl)MethylaMino]-2-pyriMidinyl]aMino]-2-Methyl-, hydrochloride | [EINECS(EC#)]
619-728-0 | [Molecular Formula]
C21H23N7O2S.ClH | [MDL Number]
MFCD12546138 | [MOL File]
635702-64-6.mol | [Molecular Weight]
473.987 |
| Chemical Properties | Back Directory | [Melting point ]
>290°C (dec.) | [storage temp. ]
Hygroscopic, Refrigerator, under inert atmosphere | [solubility ]
Acetonitrile (Slightly), DMSO (Slightly) | [form ]
Yellow powder. | [color ]
White to Off-White | [Stability:]
Hygroscopic | [InChIKey]
MQHIQUBXFFAOMK-UHFFFAOYSA-N | [SMILES]
CC1N(N=C2C=C(N(C3C=CN=C(NC4C=CC(C)=C(S(=O)(=O)N)C=4)N=3)C)C=CC=12)C.Cl |
| Hazard Information | Back Directory | [Usage]
Pazopanib (GW786034) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively-See more at: http://www.selleckchem.com/products/Pazopanib-Hyd | [Usage]
Pazopanib Hydrochloride (GW786034, Votrient, Armala) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively. | [Description]
Pazopanib Hydrochloride (635702-64-6) is the hydrochloride salt of a small molecule inhibitor of multiple protein tyrosine kinases with potential antineoplastic activity. It is an oral second-generation multitarget TKI developed by GSK and approved for marketing by the FDA in 2009 and the EMA in 2010. It targets the VEGFR, platelet-derived growth factor receptor, and c-kit, key proteins responsible for tumor growth and survival. It is used to treat patients with advanced RCC and advanced soft tissue sarcoma who have experienced chemotherapy. Pazopanib Hydrochloride has a role as an antineoplastic agent, a vascular endothelial growth factor receptor antagonist, a tyrosine kinase inhibitor, and an angiogenesis-modulating agent.
| [Originator]
GlaxoSmithKline (US) | [Uses]
Pazopanib (GW786034) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively - See more at: http://www.selleckchem.com/products/Pazopanib-Hyd | [Uses]
Pazopanib Hydrochloride (GW786034, Votrient, Armala) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively. | [Definition]
ChEBI: A hydrochloride salt prepared from equimolar amounts of pazopanib and hydrochloric acid. Used for treatment of kidney cancer. | [Brand name]
Votrient | [Clinical Use]
Pazopanib is a potent and selective multi-targeted receptor
tyrosine kinase inhibitor of VEGFR-1, VEGFR-2, VEGFR-3,
PDGFR-a/b, and c-kit that blocks tumor growth and inhibits angiogenesis.
It was approved for renal cell carcinoma by the U.S. Food
and Drug Administration in 2009 and is marketed under the trade
name Votrient by the drug’s manufacturer, GlaxoSmithKline. | [Side effects]
Pazopanib is synthesized in five chemical steps
starting from 3-methyl-6-nitroindazole, which is converted to the corresponding
2,3-dimethylindazole analog via N-methylation with trimethyloxonium
tetrafluoroborate. Subsequent reduction of the nitro group to
the amino group using tin chloride followed by condensation with 2,4dichloropyrimidine
yields a chloropyrimidinylaminoindazole intermediate.
The final two steps leading up to pazopanib consist of an N-methylation
reaction using iodomethane and cesium carbonate followed by
condensation with 5-amino-2-methylbenzenesulfonamide. | [Synthesis]
The
synthesis of pazopanib begins with methylation of 3-methyl-6-
nitroindazole (82) with trimethyl orthoformate in the presence of BF3?¤OEt to give indazole 83 in 65% yield. Reduction
of the nitro group was achieved via transfer hydrogenation to give
84 in 97% yield, and this was followed by coupling the aniline with
2,4-dichloropyrimidine in a THF-ethanol mixture at elevated
temperature to provide diarylamine 85 in 90% yield. The aniline
nitrogen was then methylated using methyl iodide to give 86 in
83% yield prior to coupling with 5-amino-2-methylbenzenesulfonamide
(87) and salt formation using an alcoholic solution of
HCl to furnish pazopanib hydrochloride (XIV) in 81% yield.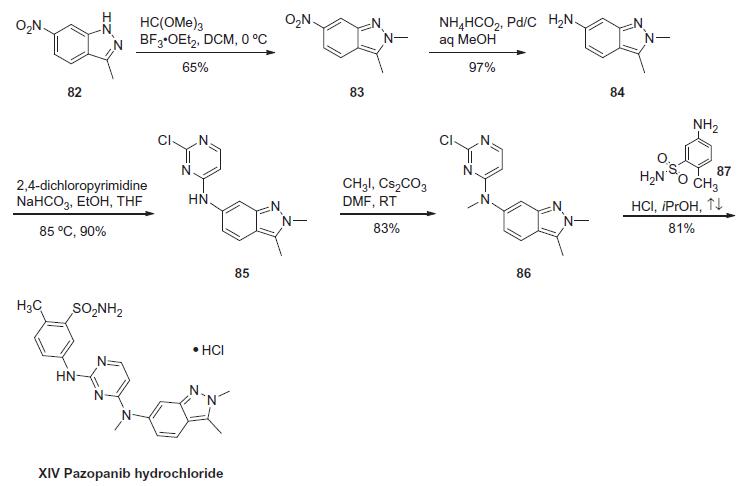
| [target]
VEGFR1 | [References]
1. verweij j, sleijfer s. pazopanib, a new therapy for metastatic soft tissue sarcoma. expert opin pharmacother 2013; 14: 929-935.2. pick am, nystrom kk. pazopanib for the treatment of metastatic renal cell carcinoma. clin ther 2012; 34: 511-520.3. bukowski rm, yasothan u, kirkpatrick p. pazopanib. nat rev drug discov 2010; 9: 17-18.4. sonpavde g, hutson te. pazopanib: a novel multitargeted tyrosine kinase inhibitor. curr oncol rep 2007; 9: 115-119.5. http://www.cancer.gov/cancertopics/druginfo/fda-pazopanibhydrochloride6. http://www.gsksource.com/gskprm/en/us/adirect/gskprm?cmd=productdetailpage&product_id=1336067580985&featurekey=603422 |
|
|