| Identification | Back Directory | [Name]
2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine | [CAS]
6713-54-8 | [Synonyms]
Homouric
Nsc20089
tetratomic
homouric acid
Dipyridamole Impurity 8
2,6,8,10-Tetrahydroxy-homopurine
Pyrimido[5,4-d]Pyrimidine Impurity 1
Pyrimido[5,4-d]pyrimidine-2,4,6,8-tetrol
pyrimido[5,4-d]pyrimidine-2,4,6,8-tetraol
pyriMido[5,4-d][1,3]diazine-2,4,6,8-tetrol
2,4,6,8-Tetrahydroxy-pynmido[5,4-d]pynmidine
,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine
2,4,6,8-TETRAHYDROXYPYRIMIDO-[5,4-D]PYRIMIDIN
2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine
1,5-dihydropyrimido[5,4-d]pyrimidine-2,4,6,8-tetrone
Pyrimido[5,4-d]pyrimidine-2,4,6,8(1H,3H,5H,7H)-tetrone
pyriMido[5,4-d]pyriMidine-2,4,6,8(1H,3H,5H,7H)-tetraone
2,4,6, 8-tetrahydroxy pyrimidine and [5, 4-D] pyrimidine
6713-54-8 2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine
1,5-dihydropyrimido[5,4-d]pyrimidine-2,4,6,8(3H,7H)tetrone
Pyrimido[5,4-d]pyrimidine-2,4,6,8(3H,7H)-tetrone, 1,5-dihydro-
1,2,3,4,5,6,7,8-Octahydropyrimido[5,4-d]pyrimidine-2,4,6,8-tetrone
1H,2H,3H,4H,5H,6H,7H,8H-[1,3]DIAZINO[5,4-D]PYRIMIDINE-2,4,6,8-TETRONE | [EINECS(EC#)]
229-766-6 | [Molecular Formula]
C6H4N4O4 | [MDL Number]
MFCD00129147 | [MOL File]
6713-54-8.mol | [Molecular Weight]
196.12 |
| Chemical Properties | Back Directory | [density ]
1.89±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C, stored under nitrogen | [pka]
5.90±0.20(Predicted) | [Appearance]
Light yellow to yellow Solid |
| Hazard Information | Back Directory | [Chemical Properties]
Crystallization. Melting point 370℃. | [Uses]
2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine is a pharmaceutical intermediate used in the preparation of Dipyridamole, an antiplatelet drug that is used primarily in the treatment of ischaemic heart disease and stroke. 2,4,6,8-Tetrahydroxy-Pyrimido-(5,4D)Pyrimidine is also used as a herbicide to control broadleaf weeds in wheat crops. | [Synthesis]
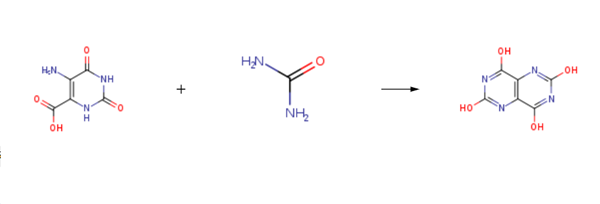
2,4,6,8-Tetrahydroxy-Pyrimido-(5,4d)Pyrimidine is prepared by the reaction of 5-amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid and urea. The specific synthesis steps are as follows:
5-amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid was charged into a two nicked flask, Urea (7.7 g, 128.0 mmol) was added dropwise with 125 ml of NaOH solution (500 g of sodium hydroxide + 1500 g of purified water) After dripping rapidly warming to 100 ° C,Insulation for one hour; continue to stir 3h,(25 ° C), filtered to obtain a solid, add purified water 250ml, heated to 60 ° C, heat lh, and then add dilute hydrochloric acid to ΡΗ = 4, cooled to room temperature filtration, washed and dried to obtain pyrimidine [5 , 4-d] pyrimidine-2,4,6,8-tetraol (tetrahydroxy), and recrystallized (ethanol solvent) to give 10.9 g in 86.9% yield.
 |
|
|