| Identification | More | [Name]
Cefotetan disodium | [CAS]
69712-56-7 | [Synonyms]
[6R-(6ALPHA,7ALPHA)]-7-[[[4-(2-AMINO-1-CARBOXY-2-OXOETHYLIDENE)-1,3-DITHIETAN-2-YL]CARBONYL]AMINO]-7-METHOXY-3-[[(1-METHYL-1H-TETRAZOL-5-YL)THIO]METHYL]-8-OXO-5-THIA-1-AZABICYCLO[4.2.0]OCT-2-ENE-2-CARBOXYLIC ACID
CEFOTETAN
CEFOTETAN DISODIUM SALT
CEFOTETAN SODIUM
disodium (7r)-7-[[4-(carbamoyl-carboxylato-methylidene)-1,3-dithietane-2-carbonyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
(6r-(6-alpha,7-alpha))--1h-tetrazol-5-yl)thio)methyl)-8-oxo
5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,7-(((4-(2-amino-1-carbox
y-2-oxoethylidene)-1,3-dithietan-2-yl)carbonyl)amino)-7-methoxy-3-(((1-methyl
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, disodium salt, [6R-(6alpha,7alpha)]-
CefotetanDisodiumC17H15N7Na2O8S4
Cefotetansodiumsalt
1,3-Dithietane, 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid deriv.
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, (6R,7S)-
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, [6R-(6α,7α)]-
Apacef
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, disodium salt, (6R,7S)-
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, disodium salt, [6R-(6α,7α)]-
Apatef
Ceftenon
Cepan Darvilen | [EINECS(EC#)]
277-834-9 | [Molecular Formula]
C17H17N7O8S4 | [MDL Number]
MFCD00864983 | [Molecular Weight]
575.62 | [MOL File]
69712-56-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
173-178°C (dec.) | [density ]
1.5157 (rough estimate) | [refractive index ]
1.7400 (estimate) | [storage temp. ]
Store at 0-5°C | [solubility ]
DMSO (Slightly), Methanol (Very Slightly) | [form ]
Solid | [pka]
1.69±0.41(Predicted) | [color ]
White to Off-White | [Merck ]
14,1934 | [Stability:]
Hygroscopic | [InChIKey]
SRZNHPXWXCNNDU-IXOPCIAXSA-N | [SMILES]
N12[C@@]([H])([C@@](NC(C3S/C(=C(\C(O)=O)/C(N)=O)/S3)=O)(OC)C1=O)SCC(CSC1N(C)N=NN=1)=C2C(O)=O | [CAS DataBase Reference]
69712-56-7(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Description]
Cefotetan is comparatively stable, lasting for approximately 24 hours at room temperature when
reconstituted. Slight yellowing and slight darkening produce materials that are still acceptable for therapy.
Cefotetan is chemically incompatible with tetracycline, aminoglycosides, and with heparin, often forming
precipitates with them. With respect to its molecular mode of action, it has a special affinity for PBP-3 of
Gram-negative bacteria, consequently producing filamentous forms. It also binds well with PBP-1A and -1B,therefore leading to cell lysis and death. Whereas it is stable to a wide range of β-lactamases, it also is a
potent inducer in some bacteria. | [Chemical Properties]
Off-White Solid | [Uses]
antibacterial | [Uses]
Cefotetan disodium is an antibiotic related to Cephalosporin, used in the treatment of bacterial infections.
| [Definition]
ChEBI: A semi-synthetic cephalosporin antibiotic with [(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl, methoxy and {[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl}amino groups at the 3, 7alpha, and 7bet
positions, respectively, of the cephem skeleton. It is resistant to a wide range of beta-lactamases and is active against a broad spectrum of aerobic and anaerobic Gram-positive and Gram-negative microorganisms. | [Brand name]
Cefotan (Zeneca). | [Antimicrobial activity]
A semisynthetic cephamycin formulated as the disodium salt
for intravenous administration. The activity is similar to that
of cefoxitin, but cefotetan exhibits more potent activity against
enterobacteria and more modest activity against Staph. aureus.
A 1 g intravenous dose achieves a serum concentration of
140–180 mg/L. There is no evidence of accumulation on a
dosage of 1 g every 12 h. Tissue fluid concentrations are about
30% of the simultaneous serum level. The plasma half-life is
about 3 h and protein binding is around 88%.
About 85% of the drug is eliminated in the urine over
24 h. Accumulation in renal failure is inversely related to
the creatinine clearance, the plasma half-life rising to 20 h
in patients requiring hemodialysis. During hemodialysis the
half-life falls to around 7.5 h and on peritoneal dialysis it falls
to 15.5 h, 5–10% of the dose being recovered in the dialysate
over 24 h.
Side effects are those typical of the group. Anaphylaxis has
been described. Because of the methylthiotetrazole side chain
there is some risk of hypoprothrombinemia, and disulfiramlike
reactions can occur. Marked changes in the bowel flora,
with appearance of C. difficile, have been reported. Uses are
similar to those of other cephamycins, but it is not widely
available. | [General Description]
Cefotetan was synthesized by Yamanouchi Pharmaceutical Co. in 1979 starting with oganomycin, a cephamycin, produced by Streptomyces oganoensis YG19Z. Its 7β side chain, 1,3-dithietan, is unique and contributes greatly to its strong activity against gramnegative bacteria, including Serratia, Citrobacter, Enterobacter, indole-positive Proteus, and anaerobes. Its half-life in the serum is as long as three hours, and about 90 % of it is excreted in the urine. | [Pharmacokinetics]
Cefotetan is a semisynthetic cephamycin antibiotic that is administered intravenously or intramuscularly. The drug is highly resistant to a broad spectrum of beta-lactamases and is active against a wide range of both aerobic and anaerobic gram-positive and gram-negative microorganisms. | [Synthesis]
Cefotetan, 7|?-[(-carbamoylcarboxylatomethylen)-1,3-dithietan-2-yl]-carboxam�ido-7-methoxy-3-(1-methyltetrazol-5-yl)-thiomethyl-3-cefem-4-carboxylic acid (32.1.2.43),
is synthesized by the following scheme. First, trisodium salt of 4-carboxy-3-hydroxy-5-mer�captoisothiazole (32.1.2.41) undergoes S-alkylation by 7|?-bromoacetamido-7|á-methoxy�cephalosporanic acid, which is synthesized by a scheme described previously (32.1.2.31) ?ú
(32.1.2.37), the only difference being that the acylation in the stage (32.1.2.35) ?ú (32.1.2.36) is accomplished not with 2-(2-thienyl)acetylchloride, but with bromoacetyl bromide. Next,
upon reacting the resulting product (32.1.2.42) with 1-methyl-1,2,3,4-tetrazol-5-thiol in the
presence of sodium bicarbonate with the expected replacement reaction, in the reaction con�ditions a ring rearrangement takes place in which the isothiazole ring is opened, and trans�formed into a derivative of carbamoylcarboxylatomethylen-1,3-dithiethane, namely cefotetan
(32.1.2.43).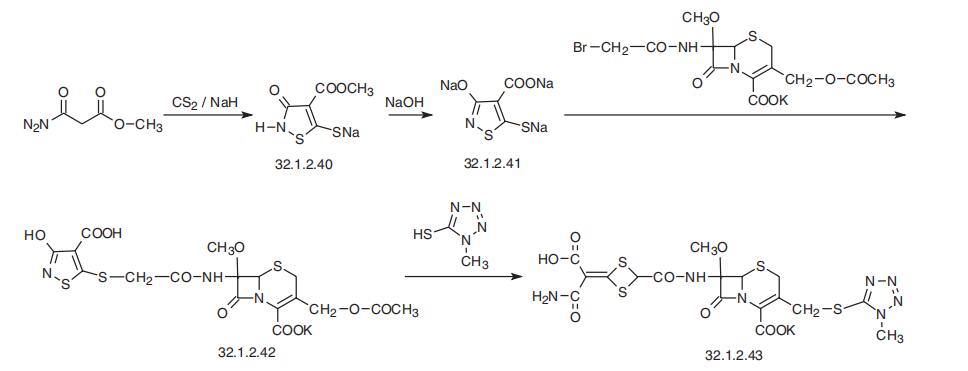
| [storage]
Store at -20°C | [Mode of action]
Cefotetan is a semi-synthetic, broad-spectrum, beta-lactamase-resistant, second-generation cephalosporin antibiotic derived from cephalosporium. The bactericidal activity of cefotetan disodium is caused by an inhibition of bacterial cell wall synthesis via inhibition of cross-linking of peptidoglycans. This results in a reduction of cell wall stability and causes cell lysis. Cefotetan disodium is more active against gram-negative organisms and less active against gram-positive organisms compared to first-generation cephalosporins. |
|
|