| Identification | More | [Name]
Hexahydrophthalic anhydride | [CAS]
85-42-7 | [Synonyms]
1,2-CYCLOHEXANEDICARBOXYLIC ANHYDRIDE
cyclohexane-1,2-dicarboxylic anhydride
HEXAHYDROPHTHALIC ANHYDRIDE
HHPA
1,2-Cyclohexanedicarboxylic Acid anhydride
1,3-Isobenzofurandione, hexahydro-
3-Isobenzofurandione,hexahydro-1
Araldite HT 907
hexahydro-3-isobenzofurandione
Lekutherm Hardener H
NT 907
Hexahydrophthalic Anhydride (HHPA)
Hexahydro benzoicanhydride
1,2-CYCLOHEXANEDICARBOXYLIC ANHYDRIDE, 9 5%
1,2-cyclohexanedicarboxylic anhydride, mixed isomers
1,2-cyclohexanedicarboxylic anhydride, predominantly cis
Hexahydro-1,3-isobenzofurandione.
CYCLOHEXANEDICARBOXYLIC ANHYDRIDE: HHPA
HEXAHYDROPHTALICACIDANHYDRIDE
Hexahydrophthalsureanhydrid | [EINECS(EC#)]
201-604-9 | [Molecular Formula]
C8H10O3 | [MDL Number]
MFCD00064863 | [Molecular Weight]
154.16 | [MOL File]
85-42-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
32-34 °C(lit.)
| [Boiling point ]
158 °C17 mm Hg(lit.)
| [density ]
1.18 | [vapor pressure ]
0.31Pa at 25℃ | [refractive index ]
1.4620 (estimate) | [RTECS ]
NP6895168 | [Fp ]
>230 °F
| [storage temp. ]
Store below +30°C. | [solubility ]
Chloroform, Methanol (Slightly) | [form ]
Solid | [pka]
4.14[at 20 ℃] | [color ]
White to Off-White | [Water Solubility ]
4.2g/L at 20℃ | [Sensitive ]
Moisture Sensitive | [BRN ]
83213 | [Exposure limits]
ACGIH: Ceiling 0.005 mg/m3 | [Stability:]
Moisture Sensitive | [InChI]
1S/C8H10O3/c9-7-5-3-1-2-4-6(5)8(10)11-7/h5-6H,1-4H2/t5-,6+ | [InChIKey]
MUTGBJKUEZFXGO-OLQVQODUSA-N | [SMILES]
O=C1OC(=O)[C@H]2CCCC[C@@H]12 | [LogP]
-4.14 at 20℃ | [Uses]
Intermediate for alkyds, plasticizers, insect repellents, and rust inhibitors; hardener in epoxy resins. | [CAS DataBase Reference]
85-42-7(CAS DataBase Reference) | [NIST Chemistry Reference]
1,2-Cyclohexane dicarboxylic anhydride(85-42-7) | [EPA Substance Registry System]
85-42-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R41:Risk of serious damage to eyes.
R42/43:May cause sensitization by inhalation and skin contact . | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24:Avoid contact with skin .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
3335 | [WGK Germany ]
1
| [Autoignition Temperature]
395°C (DIN 51794) | [TSCA ]
Yes | [HS Code ]
29172090 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Eye Dam. 1
Resp. Sens. 1
Skin Sens. 1 | [Hazardous Substances Data]
85-42-7(Hazardous Substances Data) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Tetrachlorophthalic anhydride-->2-Cyclohexene-1,2-dicarboxylic acid-->3,4,5,6-tetrahydrophthalic acid-->cis-Hexahydrophthalic acid-->3,4,5,6-Tetrahydrophthalic anhydride-->1,2,3,6-Tetrahydrophthalic anhydride | [Preparation Products]
1(3H)-Isobenzofuranone,hexahydro--->1,2-Bis(7-Methyloctyl)cyclohexyl-1,2-dicarboxylate |
| Hazard Information | Back Directory | [Hazard]
Toxic by inhalation, strong irritant to eyes and skin. | [Chemical Properties]
White crystalline powder | [Application]
Predominantly cis 1,2-cyclohexanedicarboxylic anhydride (HHPA) is a cyclic anhydride that can be used for a variety of applications such as: plasticizer, rust inhibitor, and a curing agent for epoxy based resins.
HPPA, in combination with triethaylamine (TEA), can be used as a polymerization initiator in the preparation of polyester based resins. It can also be used as a hardener to cure 1,4-butanediol diglycidyl ether which can be used as an epoxy based system for electronic devices. | [Definition]
ChEBI: A cyclic dicarboxylic anhydride that is the cyclic anhydride of hexahydrophthalic acid. | [General Description]
Predominantly cis 1,2-cyclohexanedicarboxylic anhydride (HHPA) is a cyclic anhydride that can be used for a variety of applications such as: plasticizer, rust inhibitor, and a curing agent for epoxy based resins. | [Flammability and Explosibility]
Nonflammable | [Synthesis]
Hexahydrophthalic anhydride is obtained by reacting ciscyclohexane-1, 2-dicarboxylic acid with oxalyl
chloride.Combine
ciscyclohexane-1, 2-dicarboxylic acid (1 mmol, 172 mg) and oxalyl
chloride (1.2 mmol, 152 mg, 0.103 ml) in dry toluene (5 mL) and add a
drop of freshly distilled DMF. Purge the reaction vessel with argon and
heat the reaction under stirring for 3 h. Stop the stirring, decant the
toluene solution and filter. Evaporate the volatiles. Transform into
crystalline form by trituration with diethyl ether. 1H NMR (400 MHz, CDCl3) |? 3.18 - 3.12 (m, 2H 2CH) 1.96 - 1.83 (m, 4H 2CH2) 1.57 - 1.49 (m, 4H 2CH2). HRMS (ESI), calcd for C8H10NaO3 [M+Na]+ 175.0522, found 175.0527; calcd for C9H14NaO4 [M+CH3 OH+Na]+ 209.0784, found 209.0788.
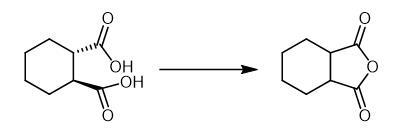
Fig The synthetic method of Hexahydrophthalic anhydride. | [Purification Methods]
It has been obtained by heating the trans-acid or anhydride at 200o. Crystallise it from *C6H6/Et2O or distil it. [Kohler & Jansen J Am Chem Soc 60 2145
1938, Abell J Org Chem 22 769 1957, Beilstein 17 II 452, 17 III/IV 5931.] |
| Questions And Answer | Back Directory | [Description]
Hexahydrophthalic anhydride (HHPA) is widely used for electronics applications, e.g. HHPA cured epoxy resins have excellent dielectric properties, high-temperature stability, and high glass transition temperatures. HHPA is used as a curing agent in adhesive coatings and sealant materials, e.g. for the second-generation two-part epoxy adhesive synthesis. Hexahydrophthalic anhydride is also used in the manufacture of alkyd and polyester resins, insecticides, and rust preventives.
| [References]
[1] Guy Rabilloud, High Performance Polymers. Vol. 1 Conductive Adhesives, 1997
[2] John Burke Sullivan and Gary R. Krieger, Clinical Environmental Health and Toxic Exposures, 2001
[3] B. A. G. Jönsson, H. Welinder, C. Hansson and B. Ståhlbom, Occupational exposure to hexahydrophthalic anhydride: air analysis, percutaneous absorption, and biological monitoring, International Archives of Occupational and Environmental Health 1993, vol. 65, 43-47
|
|
|