| Identification | Back Directory | [Name]
N,N-DIETHYL-4-METHYL-1-PIPERAZINECARBOXAMIDE | [CAS]
90-89-1 | [Synonyms]
84L
Cypip
rp3799
RP 3799
patonin
Spatonin
Notezine
Caracide
Caricide
Ethodryl
Banocide
R.P. 3799
AI3-19612
Aids007958
Bitirazine
Carbamazine
Carbilazine
Aids-007958
ditrazinebase
Ditrazine base
Carbam palatabs
5348-97-0 (Hcl)
diethlcarbamazine
1642-54-2 (Citrate)
DIETHYL CARBAMAZINE
TIMTEC-BB SBB008185
Diaethylcarbamazinum
N,N-Diethylcarbamazine
DIETHYLCARBAMAZINE USP/EP/BP
1-Diethylcarbamyl-4-methylpiperzine
1-Methyl-4-diethylcarbamylpiperazine
1-Diethylcarbamyl-4-methylpiperazine
1-DIETHYLCARBANOYL-4-METHYLPIPERAZINE
1-Methyl-4-diethylcarbamoylpiperazine
1-Diethylcarbamoyl-4-Methylpiperazine
1-(N,N-Diethylcarbamyl)-4-methylpiperazine
n,n-diethyl-4-methyl-1-piperazinecarboxamid
1-(N,N-diethylcarbamoyl)-4-methylpiperazine
N,N-DIETHYL-4-METHYL-1-PIPERAZINECARBOXAMIDE
N,N-diethyl-4-methylpiperazine-1-carboxamide
1-piperazinecarboxamide,N,N-diethyl-4-methyl-
N,N-diethyl-4-methyl-1-piperazin-4-iumcarboxamide | [EINECS(EC#)]
202-023-3 | [Molecular Formula]
C10H21N3O | [MDL Number]
MFCD00023288 | [MOL File]
90-89-1.mol | [Molecular Weight]
199.29 |
| Chemical Properties | Back Directory | [Melting point ]
65-67℃ | [Boiling point ]
127-129℃ (10 Torr) | [density ]
1.0173 (rough estimate) | [refractive index ]
1.4930 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Solid-Liquid Mixture | [pka]
pKa 7.7 (Uncertain) | [color ]
Off-white to light yellow | [EPA Substance Registry System]
Diethylcarbamazine (90-89-1) |
| Safety Data | Back Directory | [TSCA ]
TSCA listed | [HS Code ]
2933599590 | [Safety Profile]
Poison by
intraperitoneal route. Human systemic
effects by ingestion: allergc dermatitis. An
experimental teratogen. Mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx and HCl. An
additive permitted in the food and drinking
water of animals and/or for the treatment of
food-producing animals. | [Toxicity]
LD50 intraperitoneal in mouse: 240mg/kg |
| Questions And Answer | Back Directory | [Pharmacology and mechanism of action]
Diethylcarbamazine (DEC) is a piperazine derivative which was introduced in clinical medicine in 1947. The drug is active against adult and microfilariae forms of Wuchereria bancrofti, Brugia malayi, Brugia timori and Loa loa. Against Onchocerca volvulus, the drug is only effective against the microfilariae. Soon after its administration, it causes rapid disappearance of microfilariae from the blood (lymphatic filariasis) or from the skin (onchocerciasis). Its effect against microfilariae in nodules or in hydroceles and in advanced elephantiasis is minimal [1].
The mechanism of action of DEC is not well understood. The drug has no microfilaricidal effect in vitro [2]. In vivo, the microfilariae is first immobilized by the drug due to a possible hyperpolarization of the worm. This is followed by changes on the outer surface of the microfilariae making them more susceptible to the host’s defence system[3]. More recently, the drug has been reported to inhibit microtubule polymerization and disrupt preformed microtubule protein prepared from porcine brain in vitro [4]. The relevance of this action in the living parasite remains to be studied. A similar mechanism of action has been described for benzimidazoles (see Mebendazole, p. 78).
| [Indications]
DEC is used for the treatment of individual cases infected by Wuchereria bancrofti, Brugia malayi, B. timori andLoa loa. It may also be used for large scale chemotherapeutic control of filariasis. DEC is also used in Acanthocheilonema streptocerca infestations and in tropical eosinophilia.
In onchocerciasis, DEC should only be used when ivermectin is not available.
| [Side effects]
Side effects related to DEC are usually mild and include headache, general weakness, joint pains, anorexia, nausea and vomiting. They are dose-dependent. There are specific side effects seen only in patients with filariasis. They are assumed to be caused by antigens released by dying microfilariae. In lymphatic filariasis, the side effect; are usually mild. However, in onchocerciasis, the reaction to treatment with DEC may become quite severe. The reaction is known as the ‘Mazzotti’ after its original descriptior and has been used as a diagnostic test for the disease [5]. It occurs in two phases. A primary phase which commences within 24 hours and manifests as a variable combination of increased itching of the skin and eyes, photophobia, lacrimation, erythema and oedema of the skin and conjunctiva, lymphangitis, chills, anxiety, sweating and syncope. Respiratory distress, hyperpyrexia, hypotension, tachycardia and headache can also occur. Reversible proteinuria may be seen. A second phase may follow 2–6 days later with severe symmetrical acute polyarthritis predominantly in the knees, ankles, wrists, the interphalangeal joints and the shoulders. It is usually accompanied by a recrudescence of fever. The severity of Mazzotti reaction is related chiefly to the number of microfilariae killed [6].
To be able to quantify the severity of the reaction a scoring system has been developed [7]. Using this scoring system, the suppressive effects of cyproheptadine, indomethacin, prednisolone, and their combinations on the reaction were evaluated. A marked suppression of the mean total reaction score occurred only in the group treated with the full course of prednisone.
Prednisone, however, had little effect on the severity of the itching and did not prevent the occurrence of the acute febrile polyarthritis of the secondary reaction. Prednisone has also significantly impaired the therapeutic effect of DEC [8-10].
Treatment may aggravate ocular lesions and precipitate blindness as a result of the reaction against the dead and dying microfilariae. A pre-treatment eye examination is advisable in cases with a high microfilarial density in a biopsyfrom epicanthus or from other locations.
Encephalitis and retinal damage may occur in patients with loaiasis. Periarticular swellings (‘Calabar swelling’) due to a local reaction around the dying worm are also frequently seen in such patients.
| [Contraindications and precautions]
There are no known contraindications to the drug. Dosage should be reduced in patients with renal impairment[11] or in strict vegetarians with high urinary pH[12] since renal function and pH are important factors for the excretion of the drug. Dosage may also be reduced in patients in poor general condition or who are heavily infected.
| [Preparations]
Available as diethylcarbamazine citrate: 100 mg citrate is approximately equivalent to 50 mg base.
• Banocide® (Wellcome) Oral solution 10 mg/ml and 24 mg/ml; tablets 50 mg, 100 mg.
• Hetrazan® (Lederle) Tablets 50 mg.
• Notezine® (Specia) Tablets 50 mg.
| [References]
1. Webster LT (1990). Chemotherapy of parasitic infections. In: Goodman & Gilman’s The Pharmacological basis of Therapeutics, 8th edn, edited by A.G.Gilman, T.W.Rall, A.S.Nies and P. Taylor (New York: Pergamon Press) pp. 960–961.
2. Langham ME, Kramer TR (1980). The in vitro effect of diethylcarbamazine on the motility and survival of Onchocerca volvulus microfilariae. Tropenmed Parasitol, 31, 155–158.
3. Allen GD, Goodchild TM, Weatherley BC (1979). Determination of 1-diethylcarbamoyl-4methylpiperazine in human plasma and urine. J Chromatogr, 164, 521–526.
4. Edwards G, Awadzi K, Breckenridge AM, Gilles HM, L’E Orme M, Ward SA (1981). Diethylcarbamazine disposition in patients with onchocerciasis. Clin Pharmacol Ther, 30, 551–557.
5. Mazzotti L (1948). Posibilidad de utilizar como medio diagnóstico auxiliar en la oncocercosis las reacciones alérgicas consecutivas a la administración del ‘Heterzán’. Revista del Instituto Salubridad Enfermedades Tropicales, 9, 235–237.
6. Awadzi K, Gilles HM (1992). Diethylcarbamazine in the treatment of patients with onchocerciasis. Br J Clin Pharmacol, 34, 281–288.
7. Awadazi K (1980). The chemotherapy of onchocerciasis. II. Quantification of the clinical reaction to microfilaricides. Ann Trop Med Parasitol, 74, 189–197.
8. Awadzi K, Orme ML’E, Breckenridge AM, Gilles HM (1982). The chemotherapy of onchocerciasis VI. The effects of indomethacin and cyproheptadin on the Mazzotti reaction. Ann Trop Med Parasitol, 76, 323–330.
9. Awadzi K, Orme ML’E, Breckenridge AM, Gilles HM (1982). The chemotherapy of onchocerciasis VII. The effect of prednisone on the Mazzotti reaction. Ann Trop Med Parasitol, 76, 331–338.
10. Awadzi K, Orme ML’E, Breckenridge AM, Gilles HM (1982). The chemotherapy of onchocerciasis IX: The effect of prednisone plus cyproheptadine on the Mazzotti reaction. Ann Trop Med Parasitol, 76, 547–555.
11. Adjepon-Yamoah KK, Edwards G, Breckenridge AM, Orme ML’E, Ward SA (1982). The effect of renal disease on the pharmacokinetics of diethylcarbamazine in man. Br J Clin Pharmacol, 13, 829–834.
12. Edwards G, Breckenridge AM, Adjepon-Yamoah KK, Orme ML’E, Ward SA (1981). The effect of variations in urinary pH on the pharmacokinetics of diethylcarbamazine. Br J Clin Pharmacol, 12, 807–812.
|
| Hazard Information | Back Directory | [Description]
Discovered in the 1940s, diethylcarbamazine (DEC) has proven to be especially effective as a
filaricidal agent. The incidence of filariasis among American troops during World War II
necessitated a search for drugs with an antifalarial spectrum of activity. The once-popular
piperazine also was discovered during these initial screenings. Although chemically similar, the
activity again helminths is quite different. Piperazine is active against nematodes, whereas DEC is
active against falaria and microfalaria. | [Chemical Properties]
Crystals. Melting point 47-49°C, boiling point 108.5-111°C (0.4kPa). | [Originator]
Hetrazan,Lederle,US,1949 | [Definition]
ChEBI: Diethylcarbamazine is a N-methylpiperazine and a N-carbamoylpiperazine. | [Manufacturing Process]
To 50 cc of water was added 18 grams of 1-methylpiperazine dihydrochloride
and 8.34 grams of sodium hydroxide. When solution had been effected the
beaker was cooled to 10°C and with stirring, 4.17 grams of sodium hydroxide
dissolved in 15 cc of water and 14 grams of diethyl carbamyl chloride were
added simultaneously. When all had been added, the solution was extracted 3
times with ether which was then dried and filtered. The ether solution was
saturated with dry hydrogen chloride. A yellow gum appeared which on
trituration gave a white, hygroscopic solid which was filtered and dried in a
drying pistol. The N,N-diethyl-4-methyl-1-piperazine-carboxamide
hydrochloride had a melting point of 150°-155°C.
If the compound itself is desired, the salt is dissolved in water and the
solution saturated with a mild alkali such as potassium carbonate. The product
is then extracted with chloroform, dried, and after removal of the chloroform,
distilled.
| [Therapeutic Function]
Anthelmintic | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 13, p. 144, 1948 DOI: 10.1021/jo01159a019
Diethylcarbamazine | [Antimicrobial activity]
Useful activity is restricted to filarial worms. It is adulticidal
and microfilaricidal against Loa loa. Against Wuchereria bancrofti
and Brugia malayi it is predominantly microfilaricidal,
but slowly kills adult worms. It kills microfilariae, but not
adults, of Onchocerca volvulus. | [Pharmaceutical Applications]
A carbamyl derivative of piperazine formulated as the citrate.
It is readily soluble in water and slightly hygroscopic. | [Mechanism of action]
Although studied extensively, the mechanism of action of DEC remains unknown.
Diethylcarbamazine appears to be the active form of the drug, with a very rapid onset of action
(within minutes), but of interest is the fact that the drug is inactive in vitro, suggesting that
activation of a cellular component is essential to the filaricidal action. Three mechanisms have
been suggested. The first is involvement of blood platelets triggered by the action of filarial
excretory antigens. A complex reaction is thought to occur between the drug, the antigen, and
platelets. Although these authors were unable to show a direct action of the drug on the
microfalaria, a more recent study showed that DEC produced morphological damage to the
microfalaria. The damage consisted of the loss of the cellular sheath, exposing antigenic
determinants to immune defense mechanisms. Severe damage then occurred to microfalaria
organelles, leading to death. The second is inhibition of microtubule polymerization and
disruption of preformed microtubules. The third is interference with arachidonic acid
metabolism. Diethylcarbamazine is known to have anti-inflammatory action, which appears to
involve blockage at cyclooxygenase and leukotriene A4 synthase (leukotriene synthesis). This
action appears to alter vascular and cellular adhesiveness and cell activation. This latter action
would suggest a possible relationship between the first and third mechanism. | [Pharmacokinetics]
Oral absorption: >90%
Cmax 200 mg: 1.5–2 mg/L after 2 h
Plasma half-life: c. 6–12 h
Volume of distribution: 107–371 L
Plasma protein binding: Very low
Like piperazine (to which it is related), diethylcarbamazine
is rapidly and completely absorbed. About half the dose is
excreted unchanged in the urine; the rest is metabolized and
eliminated by renal and extrarenal routes. | [Clinical Use]
Filariasis
It has also been used for visceral larva migrans, but experience
is limited and there is little evidence of its efficacy. | [Synthesis]
Diethylcarbamazine, N, N-diethyl-4-methyl-1-piperazincarboxamide
(38.1.13), is made by acylating 1-methylpiperazine with diethylcarbamoylchloride.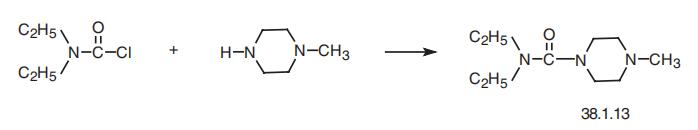
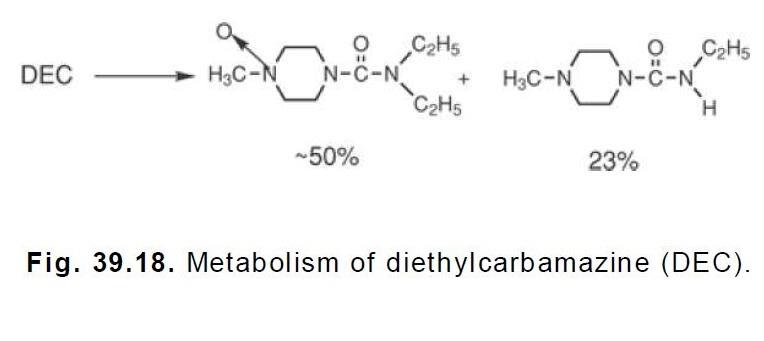
| [Metabolism]
The metabolism of DEC leads to the compounds shown in Figure 39.18 plus trace amounts of
methylpiperazine and piperazine. Nearly all of the metabolites appear in the urine. As much as 10
to 20% of the drug is excreted unchanged. As indicated by the rapid action of the drug, it would
appear that none of the metabolites are involved in the therapeutic action of DEC.
 |
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|