101184-73-0
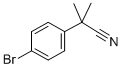 101184-73-0 结构式
101184-73-0 结构式
基本信息
Α,Α-二甲基对溴苯基乙腈
2-(4-溴苯基)-2-甲基丙腈
2-(4-BROMOPHENYL)-2-METHYLPROPIONITRILE
4-Bromo-alpha,alpha-dimethylbenzeneacetonitrile
P-Bromo a,a-Dimethyl Phenyl Acetonitrile
2-(4-Bromophenyl)-2-methylpropanenitrile 98%
ALPHA,ALPHA-DIMETHYL-4-BROMOPHENYLACETONITRILE
α,α-Dimethyl 4-bromophenylacetonitrile
制备方法
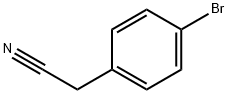
16532-79-9

74-88-4

101184-73-0
在室温条件下,将4-溴苯基乙腈(10.0 g,51.0 mmol)溶解于四氢呋喃(200 mL)中,制备成溶液。随后,将该溶液缓慢滴加至氢化钠(60%矿物油分散体,6.0 g,153 mmol)的四氢呋喃(400 mL)悬浮液中,滴加过程持续30分钟。滴加完毕后,在30分钟内缓慢加入碘甲烷(7.6 mL,122 mmol),期间偶尔使用水浴冷却以维持反应温度低于40℃。反应混合物在室温下搅拌过夜。反应完成后,将混合物倒入水(500 mL)中,并用乙酸乙酯(2×300 mL)进行萃取。合并有机层,依次用盐水(2×300 mL)洗涤,无水硫酸镁干燥,过滤后浓缩溶剂。粗产物通过硅胶柱色谱法纯化,以含2%乙酸乙酯的己烷溶液为洗脱剂,得到2-(4-溴苯基)-2-甲基丙腈(11 g,收率96%),为黄色油状物。1H NMR(300 MHz,CDCl3)δ ppm:1.7(s,6H),7.35(dd,2H),7.53(dd,2H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2008, vol. 51, # 24, p. 7953 - 7967
[2] Patent: US2009/12123, 2009, A1. Location in patent: Page/Page column 5
[3] Patent: US2010/197591, 2010, A1. Location in patent: Page/Page column 29
[4] Patent: EP1870099, 2007, A1. Location in patent: Page/Page column 7-8
[5] Patent: US2012/108574, 2012, A1. Location in patent: Page/Page column 111

