103285-22-9
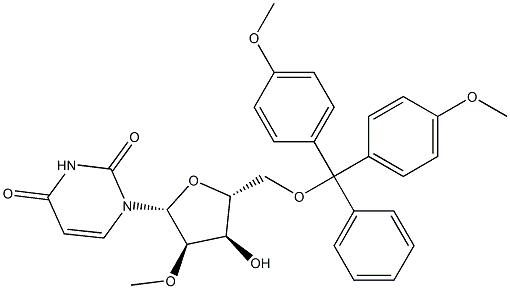 103285-22-9 结构式
103285-22-9 结构式
基本信息
5'-O-(4,4'-二甲氧基三苯基甲基)-2'-甲氧基尿苷
5'-DMT-2'-OMe-U
DMT-2'-O-METHYLURIDINE
5'-O-DMT-2'-O-methyluridine
5'-O-DMT-2'-O-methyl-D-uridine
5'-O-(DIMETHOXYTRITYL)-2'-O-METHYLURIDINE
5'-O-(4,4'-DIMETHOXYTRITYL)-2'-O-METHYLURIDINE
5'-O-[Bis(4-methoxyphenyl)phenylmethyl]-2'-O-methyl-uridine
Uridine, 5'-O-[bis(4-Methoxyphenyl)phenylMethyl]-2'-O-Methyl-
1-((2R,3R,4R,5R)-5-((bis(4-Methoxyphenyl)(phenyl)Methoxy)Methyl)-4-hydroxy-3-Methoxytetrahydrofuran-2-yl)pyriMidine-2,4(1H,3H)-dione
物理化学性质
制备方法
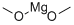
109-88-6
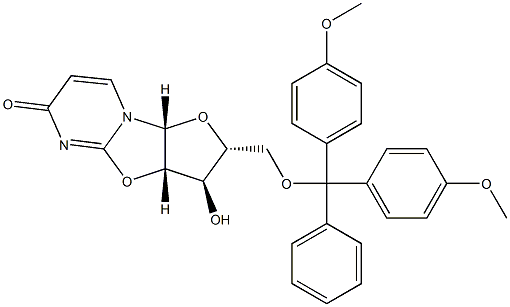
173170-12-2

103285-22-9
甲醇镁和N/A为原料合成1-((2R,3R,4R,5R)-5-((双(4-甲氧基苯基)(苯基)甲氧基)甲基)-4-羟基-3-甲氧基四氢呋喃-2-基)嘧啶-2,4(1H,3H)-二酮的一般步骤如下:按照文献方法,从尿苷经两步反应制备5'-O-DMT-2,2'-O-脱水尿苷(6)。随后,参照文献报道的方法,将化合物6转化为5'-O-DMT-2'-O-甲基尿苷。具体操作如下:首先,将镁粉(0.5g)在甲醇(50mL)中于60℃加热2小时,蒸发过量甲醇并在真空下干燥,得到灰色粉末状的甲醇镁。将干燥的甲醇镁(740mg,8.57mmol,6.00当量)加入化合物6(750mg,1.42mmol,1.00当量)在DMF(20mL)中的悬浮液中,将混合物加热至100℃反应2小时,直至溶液澄清。反应完成后,蒸发除去溶剂,将残余物溶解于乙酸乙酯中。有机层依次用NaHCO3溶液和水洗涤,无水Na2SO4干燥后减压浓缩。粗产物通过硅胶柱色谱法纯化,洗脱剂为含2-4%甲醇的二氯甲烷溶液,得到化合物7(510mg,0.91mmol,收率64%)为白色固体。1H NMR(400MHz,CDCl3):δ(ppm)= 3.54-3.57(m,2H,H-C(5')),3.65(s,3H,2'-OCH3),3.79-3.80(m,7H,H-C(2'),DMT-OCH3),3.98-4.01(m,1H,H-C(4')),4.45-4.50(m,1H,H-C(3')),5.26(d,1H,J = 8.1 Hz,H-C(5)),5.97(s,1H,H-C(1')),6.82-6.86(m,4H,DMT-Har),7.24-7.39(m,9H,DMT-Har),8.03(d,1H,J = 8.1Hz,H-C(6));MS(ESI):m/z = 583.1 [M + Na]+。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 20, p. 6171 - 6180
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-W025438 | 5’-O-(4,4’-Dimethoxytrityl)-2’-O-methyluridine | 103285-22-9 | 1 g | 200元 |
| 2025/12/22 | HY-W025438 | DMT保护性-2'-甲氧基尿苷 5’-O-(4,4’-Dimethoxytrityl)-2’-O-methyluridine | 103285-22-9 | 10 mM * 1 mLin DMSO | 220元 |
| 2025/12/22 | HY-W025438 | DMT保护性-2'-甲氧基尿苷 5’-O-(4,4’-Dimethoxytrityl)-2’-O-methyluridine | 103285-22-9 | 5 g | 450元 |