110567-22-1
 110567-22-1 结构式
110567-22-1 结构式
基本信息
物理化学性质
制备方法

100-39-0
![(1S,2R,3S,5R)-2-[(苄氧甲基)-6-氧杂二环[3.1.0]己-3-醇](/CAS/GIF/117641-39-1.gif)
117641-39-1
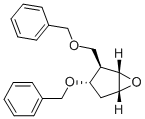
![(1S,2R,3S,5R)-3-(苄氧甲基)-6-氧杂二环[3.1.0]己烷](/CAS/20180808/GIF/110567-22-1.gif)
110567-22-1
一般步骤:将氢化钠(55-65%,0.93 kg,21.3-25.2 mol)悬浮于无水四氢呋喃(25 kg)中,室温搅拌10分钟。在20-30℃下缓慢滴加(1S,2R,3S,5R)-2-((苄氧基)甲基)-6-氧杂双环[3.1.0]己烷-3-醇(2.99 kg,12.3 mol)的四氢呋喃溶液。随后,在室温下于2-3小时内逐滴加入苄基溴(3.14 kg,18.3 mol)。反应完成后,加入无水乙醇(1.5 kg)淬灭反应,并在10℃下搅拌。减压蒸馏除去溶剂,残余物用乙酸乙酯溶解。所得溶液用饱和食盐水(10 kg,3次)洗涤,有机相经无水硫酸钠干燥后,减压浓缩,得到(1S,2R,3S,5R)-3-(苯甲氧基)-2-[(苯甲氧基)甲基]-6-氧杂双环[3.1.0]己烷(4.4 kg)为红褐色油状物。1H NMR (400 MHz, DMSO-d6): δ 1.923-1.960 (1H, m), 2.026-2.087 (1H, m), 2.448-2.436 (1H, m), 3.324-3.520 (3H, m), 3.520 (1H, s), 3.865-3.884 (1H, m), 4.322-4.391 (2H, m), 4.480 (2H, s), 7.251-7.373 (10H, m). HRMS (ESI): m/z calcd for C20H22O3Na [M+Na]+ 333.1467, found 333.1463.
参考文献:
[1] Synthesis, 2003, # 13, p. 2101 - 2109
[2] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1988, p. 549 - 554
[3] Bioorganic and Medicinal Chemistry Letters, 1997, vol. 7, # 2, p. 127 - 132
[4] Journal of Labelled Compounds and Radiopharmaceuticals, 2009, vol. 52, # 11, p. 485 - 489
[5] Organic Preparations and Procedures International, 2017, vol. 49, # 6, p. 568 - 574
