111-58-0
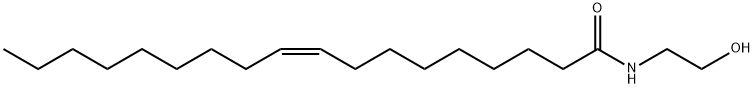 111-58-0 结构式
111-58-0 结构式
基本信息
油酰乙醇胺
油酰胺 MEA
油酰单乙醇胺
ODA
OLEIC ACID-2,6-DIISOPROPYL ANILIDE
N-(2-hydroxyethyl)-,(Z)-9-Octadecenamide
Oleoylmonoethanolamide
OLEAMIDE MEA
9-Octadecenamide, N-(2-hydroxyethyl)-, (9Z)-
(Z)-N-(2-hydroxyethyl)octadec-9-enamide
LSUREMONOETHANOLAMID
Oleyl monoethanolamide
N-(2-Hydroxyethyl)oleamide
N-(cis-9-Octadecenoyl)ethanolamine
N-Oleoylethanolamine
OEA
N-(cis-9-Octadecenoyl)ethanolamine, N-(Hydroxyethyl)oleamide, OEA, oleoylethanolamide
(Z)-N-(2-Hydroxyethyl)-9-octadecenamide
AM-3101
N-(2-Hydroxyethyl)oleic amide
物理化学性质
ANTISTATIC
SURFACTANT - FOAM BOOSTING
安全数据
制备方法
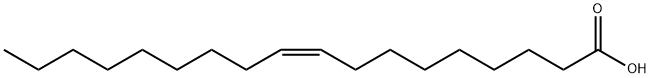
112-80-1

141-43-5
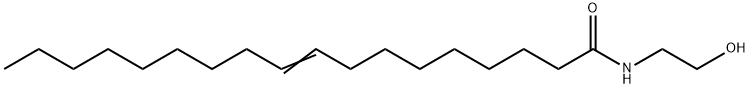
7545-20-2
传统合成脂肪酸乙醇酰胺的方法常导致产物出现不理想的颜色和气味,尽管已有研究提出通过添加除臭剂和抗氧化剂来改善产品质量(Kolancilar, J.Am.Oil.Chem.Soc.81:597-98, 2004; Bilyk et al., J.Am.Oil.Chem.Soc.69:488-91, 1992; Tufvesson et al., Biotechnol.Bioeng.97:447-53, 2007)。本实施例比较了在脂肪酶催化下,使用过量乙醇胺作为溶剂与使用己烷作为溶剂合成油酰乙醇酰胺的可行性。研究表明,当以三酰基甘油作为酰基供体时,采用过量乙醇胺作为溶剂能有效合成脂肪酸乙醇酰胺(Kolancilar, J.Am.Oil.Chem.Soc.81:597-98, 2004; Bilyk et al., J.Am.Oil.Chem.Soc.69:488-91, 1992)。
参考文献:
[1] Patent: US2013/303795, 2013, A1. Location in patent: Paragraph 0142; 0143; 0152; 0153
[2] Chemical and Pharmaceutical Bulletin, 2015, vol. 63, # 4, p. 278 - 285
[3] Chemistry and Physics of Lipids, 2012, vol. 165, # 7, p. 705 - 711
[4] Letters in Organic Chemistry, 2009, vol. 6, # 6, p. 444 - 447
[5] Patent: US1990453, 1933,
常见问题列表
|
Human Endogenous Metabolite
|
PPAR-α
|
Oleoylethanolamide (OEA), an endogenous PPAR-α ligand, attenuates liver fibrosis targeting hepatic stellate cells. Oleoylethanolamide suppresses TGF-β1 induced hepatic stellate cells (HSCs) activation in vitro via PPAR-α. To assess the impact of Oleoylethanolamide on HSCs activation, the expression levels of α-SMA and Col1a in TGF-β1-stimulated HSCs are examined by qPCR. The mRNA levels of α-SMA and Col1a are markedly induced in the group of CFSC cells with TGF-β1 (5 ng/mL) stimulation for 48h, while the mRNA levels are suppressed when treated with Oleoylethanolamide in a dose-dependent manner. Immunofluorescence and western blot results show that Oleoylethanolamide treatment dose-dependently inhibits the protein expression of α-SMA, the marker of HSC activation. The inhibitory effects of Oleoylethanolamide on HSCs activation are completely blocked by PPAR-α antagonist MK886 (10 μM). Moreover, the mRNA and protein expression levels of PPAR-α are down-regulated with TGF-β1 stimulation, while Oleoylethanolamide treatment restores these changes in dose-dependent manner. In addition, the phosphorylation of Smad 2/3 is upregulated in the presence of TGF-β1 stimulation, consistent with the observed effects on HSC activation, while Oleoylethanolamide (10 μM) reduces the phosphorylation of Smad2/3 in CFSC simulated with TGF-β1.
Oleoylethanolamide (OEA) can significantly suppress the pro-fibrotic cytokine TGF-β1 negatively regulate genes in the TGF-β1 signaling pathway (α-SMA, collagen 1a, and collagen 3a) in mice models of hepatic fibrosis. Treatment with Oleoylethanolamide (5 mg/kg/day, intraperitoneal injection, i.p.) significantly attenuates the progress of liver fibrosis in both two experimental animal models by blocking the activation of hepatic stellate cells (HSCs).

