116026-98-3
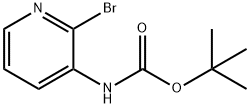 116026-98-3 结构式
116026-98-3 结构式
基本信息
(2-溴吡啶-3-基)氨基甲酸叔丁酯
(2-溴-3-吡啶基)氨基甲酸叔丁酯
3-(BOC-AMINO)-2-BROMOPYRIDINE
2-BROMO-3-N-BOC-AMINOPYRIDINE
TERT-BUTYL 2-BROMOPYRIDIN-3-YLCARBAMATE
tert-butyl N-(2-broMopyridin-3-yl)carbaMate
tert-Butyl 2-bromopyridin-3-ylcarbamate ,97%
2-bromo-3-[N-(t-butyloxycarbonylamino]pyridine
2-Bromo-3[N-(tert-butyloxycar-bonyl)amino]pyridine
N-(2-bromo-3-pyridinyl)carbamic acid tert-butyl ester
(2-BROMO-PYRIDIN-3-YL)-CARBAMIC ACID TERT-BUTYL ESTER
物理化学性质
安全数据
制备方法
39856-58-1

24424-99-5

116026-98-3
步骤1:2-溴吡啶-3-基氨基甲酸叔丁酯(10a)的合成 向2-溴吡啶-3-胺(3.0g,17.3mmol)的四氢呋喃(THF,30mL)溶液中,于0℃下缓慢加入双(三甲基甲硅烷基)氨基化锂(1M,35mL,35mmol)。将反应混合物在该温度下搅拌30分钟。随后,加入二碳酸二叔丁酯(Boc2O,3.9g,17.3mmol)的THF(20mL)溶液,并将混合物在室温下搅拌1.5小时。反应完成后,将反应混合物倒入0.1M盐酸(HCl)中,并用乙酸乙酯(EA)萃取。合并有机层,用饱和食盐水(盐水)洗涤,无水硫酸钠干燥,减压浓缩。粗产物通过柱层析(CC,石油醚/乙酸乙酯=5/1)纯化,得到目标化合物10a(2.9g,62%收率),为白色固体。
参考文献:
[1] Journal of Medicinal Chemistry, 2009, vol. 52, # 16, p. 5032 - 5043
[2] Patent: WO2013/64231, 2013, A1. Location in patent: Page/Page column 51
[3] Patent: US2013/131036, 2013, A1. Location in patent: Paragraph 1676; 1677
[4] Patent: WO2013/62966, 2013, A2. Location in patent: Paragraph 203; 204
[5] Patent: WO2005/21529, 2005, A1. Location in patent: Page/Page column 31
