1174046-72-0
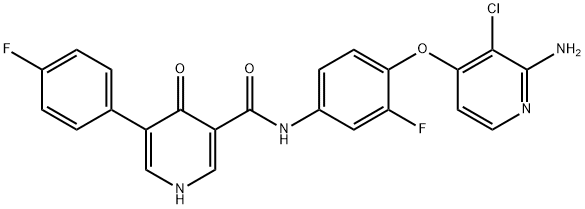 1174046-72-0 结构式
1174046-72-0 结构式
基本信息
N-[4-[(2-氨基-3-氯-4-吡啶)氧基]-3-氟苯基]-5-(4-氟苯基)-1,4-二氢-4-氧代-3-吡啶羧酰胺
N-[4-((2-氨基-3-氯吡啶-4-基)氧基)-3-氟苯基]-5-(4-氟苯基)-4-氧代-1,4-二氢吡啶-3-甲酰胺
CS-1738
MS-794833
BMS-794833
BMS-794833 USP/EP/BP
PDYXPCKITKHFOZ-UHFFFAOYSA-N
N-[4-(2-AMINO-3-CHLOROPYRIDIN-4-YL)OXY-3-FLUOROPHENYL]-5-(4-FLUOROPHENYL)-4-OXO-1H-PYRIDINE-3-CARBOXAMIDE
N-[4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl]-5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide
N-[4-((2-Amino-3-chloropyridin-4-yl)oxy)-3-fluorophenyl]-5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide
3-PyridinecarboxaMide, N-[4-[(2-aMino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-5-(4-fluorophenyl)-1,4-dihydro-4-oxo-
物理化学性质
制备方法
![3-Pyridinecarboxamide, N-[4-[[3-chloro-2-[(diphenylmethylene)amino]-4-pyridinyl]oxy]-3-fluorophenyl]-5-(4-fluorophenyl)-1,4-dihydro-4-oxo-](/CAS/20200611/GIF/1174047-03-0.gif)
1174047-03-0

1174046-72-0
以化合物(CAS:1174047-03-0)为原料合成N-(4-((2-氨基-3-氯吡啶-4-基)氧基)-3-氟苯基)-5-(4-氟苯基)-4-氧代-1,4-二氢吡啶-3-甲酰胺的一般步骤:在室温下,将N-(4-(3-氯-2-(二苯基亚甲基氨基)吡啶-4-基氧基)-3-氟苯基)-5-(4-氟苯基)-4-氧代-1,4-二氢吡啶-3-甲酰胺(410mg,0.65mmol)的THF(10mL)溶液缓慢加入2M HCl水溶液(0.81mL,1.62mmol)中。反应混合物在室温下搅拌1小时后,进行真空浓缩。将残余物冷却至室温后,加入5%的冷盐酸水溶液(5mL)。通过过滤收集析出的固体,依次用水和乙醚洗涤,最后在真空下干燥,得到目标产物(275mg,收率90%)。产物经1H NMR(DMSO-d6)和质谱(ESI+)确认:1H NMR (DMSO-d6) δ 13.31 (s, 1H), 12.70 (br s, 1H), 8.63 (d, 1H, J = 1.30 Hz), 8.09 (d, 1H, J = 1.50 Hz), 8.02 (dd, 1H, J = 2.50, 13.10 Hz), 7.76 (d, 1H, J = 5.50 Hz), 7.71 (m, 2H), 7.44 (dd, 1H, J = 1.50, 8.80 Hz), 7.31 (t, 1H, J = 8.80 Hz), 7.27 (t, 2H, J = 8.80 Hz), 6.43 (br s, 2H), 5.96 (d, 1H, J = 5.60 Hz); MS (ESI+) m/z 469 (M + H)+。
参考文献:
[1] Patent: WO2009/94417, 2009, A1. Location in patent: Page/Page column 27; 37
常见问题列表
| Target | Value |
| Met | 1.7 nM |
| VEGFR2 | 15 nM |
BMS794833也抑制Met受体激活的胃癌细胞系GTL-16, IC50 为39 nM。