120511-84-4
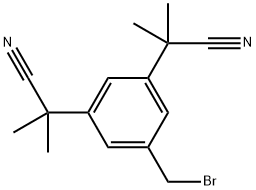 120511-84-4 结构式
120511-84-4 结构式
基本信息
5-溴甲基-a,a,a',a'-四甲基-1,3-二乙氰基苯
5-溴甲基-四甲基-1,3-二乙氰基苯
阿那曲唑中间体 2
3,5-Bis(2-cyanoprop-2-yl)benzyl bromide
5-(BROMOMETHYL)-A,A,A,A-TETRAMETHYL-1,3-BENZENEDIACETONITRILE
5-BROMOMETHYL-A,A,A',A'-TETRAMETHYL-1,3-BENZENEDIACETONITRILE
5-(BROMOMETHYL)-ALPHA,ALPHA,ALPHA,ALPHA-TETRAMETHYL-1,3-BENZENEDIACETONITRILE
5-BROMOMETHYLTETRAMETHYL-1,3-BENZENEDIACETONITRILE
2,2'-(5-BROMOMETHYL-1,3-PHENYLENE)-DI-(2-METHYLPROPIONITRILE)
5-BROMOMETHYL-A,A,A',A'-TETRAMETHYL-1,3-BENZENEDIACETONITRIL
3,5-Bis(1-Cyano-1-Methylethyl)BromomethylBenezene(ForAnastrozole)
3,5-Bis(1-cyano-1-methylethyl)bromomethylbenezene
forAnastrozole
5-(BROMOMETHYI)-A,A,A'',A''-TETRAMETHYI-1,3-BENZENEDIACETONITRILE
2-[3-(Cyanodimethylmethyl)-5-bromomethylphenyl]-2-methylpropionitrile
5-(bromomethyl)-α,α,α',α'-tetramethyl-
2-[3-(Bromomethyl)-5-(1-cyano-1-methylethyl)phenyl]-2-methylpropanenitrile
物理化学性质
制备方法
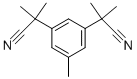
120511-72-0

120511-84-4
以5,α,α,α',α'-五甲基-1,3-苯二乙腈为原料,经过步骤III得到3,5-双(1-氰基-1-甲基乙基)甲苯(600g,2.65mol)。将该产物与N-溴代琥珀酰亚胺(519g,2.916mol)和过氧化苯甲酰(17g,0.053mol)混合于四氯化碳(4.5L)中,回流反应2至2.5小时。反应完成后,冷却反应混合物并过滤。滤液在40℃至45℃下浓缩至干燥。所得固体在75℃至80℃下溶解于2-丙醇(2L)中,随后缓慢冷却至10℃至15℃,并在此温度下搅拌1小时。进一步将反应混合物冷却至-5℃至0℃,继续搅拌2小时,之后过滤,并用预冷的2-丙醇(200ml)洗涤滤饼。滤饼随后用正己烷洗涤并吸干。最后,将滤饼在40℃至45℃下真空干燥,得到目标产物3,5-双(1-氰基-1-甲基乙基)苄基溴(700.2g,收率87.0%)。
参考文献:
[1] Patent: US2006/189670, 2006, A1. Location in patent: Page/Page column 5; 7
[2] Patent: US2006/35950, 2006, A1. Location in patent: Page/Page column 3; 11
[3] Patent: CN103554041, 2016, B. Location in patent: Paragraph 0014-0016
[4] Patent: US2009/221837, 2009, A1. Location in patent: Page/Page column 4
[5] Patent: WO2007/39913, 2007, A1. Location in patent: Page/Page column 14
