1346574-57-9
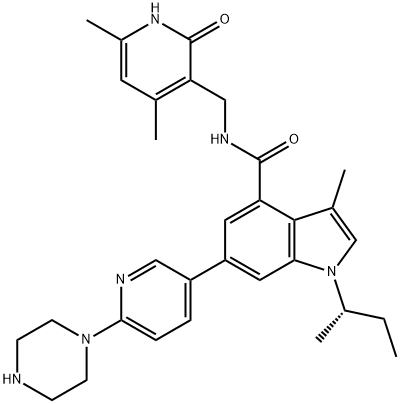 1346574-57-9 结构式
1346574-57-9 结构式
基本信息
CPDB1065
GSK126
GSK-126
GSK 126
GSK126, >=98%
N-[(4,6-Dimethyl-2-ox
1-(S)-sec-butyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-methyl-6-(6-(piperazin-1-yl)pyridin-3-yl)-1H-indole-4-carboxamide
S)-1-(sec-butyl)-N-((4,6-diMethyl-2-oxo-1,2-dihydropyridin-3-yl)Methyl)-3-Methyl-6-(6-(piperazin-1-yl)pyridin-3-yl)-1H-indole-4-carboxaMide
N-[(4,6-Dimethyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-3-methyl-1-((1S)-1-methylpropyl)-6-[6-(1-piperazinyl)-3-pyridinyl]-1H-indole-4-carboxamide
N-[(4,6-Dimethyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-3-methyl-1-((1S)-1-methylpropyl)-6-[6-(1-piperazinyl)-3-pyridinyl]-1H-indole-4-carboxamide GSK126
GSK 126 N-[(4,6-Dimethyl-2-oxo-1,2-dihydro-3-pyridinyl)methyl]-3-methyl-1-((1S)-1-methylpropyl)-6-[6-(1-piperazinyl)-3-pyridinyl]-1H-indole-4-carboxamide
物理化学性质
制备方法
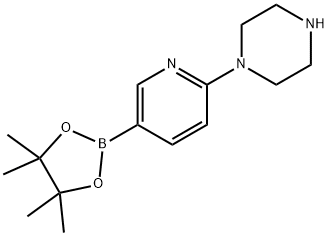
871125-86-9
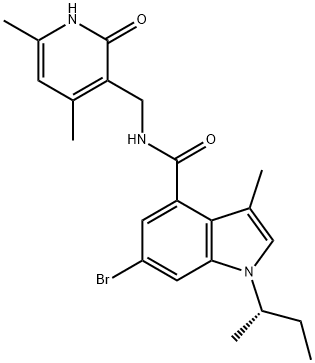
1346574-54-6

1346574-57-9
以2-(哌嗪-1-基)吡啶-5-硼酸频那醇酯和(S)-6-溴-1-(仲丁基)-N-((4,6-二甲基-2-氧代-1,2-二氢吡啶-3-基)甲基)-3-甲基-1H-吲哚-4-甲酰胺为原料,合成(S)-1-(仲丁基)-N-((4,6-二甲基-2-氧代-1,2-二氢吡啶-3-基)甲基)-3-甲基-6-(6-(哌嗪-1-基)吡啶-3-基)-1H-吲哚-4-甲酰胺的一般步骤如下:在100 mL三颈烧瓶中加入(S)-6-溴-1-(仲丁基)-N-((4,6-二甲基-2-氧代-1,2-二氢吡啶-3-基)甲基)-3-甲基-1H-吲哚-4-甲酰胺(365 mg,0.82 mmol)、2-(哌嗪-1-基)吡啶-5-硼酸频那醇酯(309 mg,1.07 mmol,1.3当量)、磷酸钾(522 mg,2.46 mmol,3当量)、水以及1,4-二氧六环溶剂。随后,在氮气保护下加入[1,1'-双(二苯基膦基)二茂铁]二氯化钯(II)二氯甲烷络合物(53.9 mg,0.066 mmol),并将反应混合物加热至90℃进行反应。反应完成后,经过纯化处理,得到目标产物400 mg,收率为92%。
参考文献:
[1] Patent: CN105541801, 2016, A. Location in patent: Paragraph 0118
[2] Synthetic Communications, 2016, vol. 46, # 14, p. 1215 - 1222
[3] Patent: WO2011/140324, 2011, A1. Location in patent: Page/Page column 83-84
[4] Patent: US2014/256739, 2014, A1. Location in patent: Paragraph 0566
常见问题列表
| Target | Value |
|
EZH2
(Cell-free assay) | 9.9 nM |
在体外,EZH2野生型和突变型DLBCL细胞系中,GSK126最有效地抑制H3K27me3,其次是H3K27me2。GSK126也能有效抑制EZH2突变型DLBCL细胞系的增殖,并诱导敏感细胞系中EZH2靶基因的转录激活。在A687V EZH2突变细胞中,GSK126处理导致总体H3K27me3减少,强基因活化,胱天蛋白酶活化,以及增殖减少。在亲代H2087细胞中,GSK126抑制VEGF-A和磷酸化Ser(473)-AK的表达,因此引起对细胞增殖,迁移和代谢的抑制。
