139756-02-8
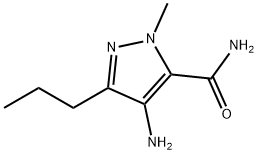 139756-02-8 结构式
139756-02-8 结构式
基本信息
4-氨基-1-甲基-3-正丙基-1H(氢)-吡唑-5-羧酰胺
4-氨基-1-甲基-3-正丙基-1H-吡唑-5-羧酰胺
西地那非中间体
4-AMINO-1-METHYL-3-N-PROPYL-1H-PYRAZOLE-5-CARBOXAMIDE
4-AMINO-1-METHYL-3-N-PROPYL-5-PYRAZOLECARBOXAMIDE
4-AMINO-1-METHYL-3-N-PROPYL PYRAZOLE-5-CARBOXAMIDE
4-AMINO-1-METHYL-3-N-PROPYL PYRAZOLO-5-CARBOXAMIDE
4-AMINO-1-METHYL-3-N-PROPYPRZOLE-5-CARBOXAMIDE
4-AMINO-1-METHYL-3-PROPYL-1H-PYRAZOLE-5-CARBOXAMIDE
4-amino-1-methyl-3-propyl-5-pyrazolecarboxamide
4-AMINO-1-METHYL-3-PROPYL PYRAZOLE-5-CARBOXAMIDE
4-Amino-2-methyl-5-propyl-2H-pyrazole-3-carboxamide
4-AMINO-2-METHYL-5-PROPYL-2H-PYRAZOLE-3-CARBOXYLIC ACID AMIDE
VIAGRA INTERMEDIATE
1H-Pyrazole-5-carboxamide,4-amino-1-methyl-3-n-propyl
4-AMINO-1-METHYL-3-N-PROPYL-5-PYRAZOLECA
1H-Pyrazole-5-carboxamide,4-am
4-Amino-1-methyl-3-propyl-5-pyrazolecarboxam
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
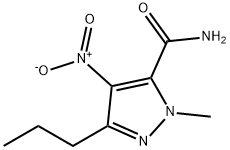
139756-01-7

139756-02-8
步骤7 4-氨基-1-甲基-3-正丙基-1H-吡唑-5-甲酰胺的合成:将1-甲基-4-硝基-3-丙基-1H-吡唑-5-甲酰胺(14.7g,69.3mmol)悬浮于乙酸乙酯(130mL)中,加入10%钯/碳催化剂(3.3g)。在50℃和4atm氢气压力下,将反应混合物搅拌过夜。反应完成后,冷却至室温,过滤除去催化剂,并用乙酸乙酯洗涤催化剂。合并的滤液用无水硫酸镁干燥,过滤后减压浓缩,得到白色固体4-氨基-1-甲基-3-正丙基-1H-吡唑-5-甲酰胺(13.8g,收率98%)。产物经1H NMR(300MHz,CDCl3)确认:δ4.12(s,3H),2.84(s,2H),2.55(t,2H,J = 7.2Hz),1.71-1.61(m,2H),0.99(t,3H,J = 7.2Hz);LC-MS显示m/z = 183(MH)+。
参考文献:
[1] Patent: US2008/194529, 2008, A1. Location in patent: Page/Page column 57
[2] Patent: WO2014/131855, 2014, A1. Location in patent: Page/Page column 81
[3] Patent: WO2016/20307, 2016, A1. Location in patent: Page/Page column 51
[4] Patent: CN103044330, 2018, B. Location in patent: Paragraph 0025; 0031-0032; 0034-0035
[5] Patent: WO2015/114647, 2015, A1. Location in patent: Page/Page column 47; 48
