1517-82-4
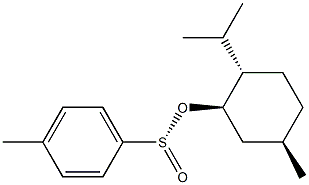 1517-82-4 结构式
1517-82-4 结构式
基本信息
(S)-(-)-薄荷基 对甲苯亚磺酸酯
(-)-薄荷醇 (S)-甲苯-4-亚磺酸酯
(-)-(1R)-薄荷基 (S)-对甲苯亚磺酸酯
(1R,2S,5R)-(-)-薄荷醇(S)-对甲苯磺酸
(1R,2S,5R)-(-)-孟基 (S)-对甲苯亚磺酸酯
(1R,2S,5R)-(-)-薄荷基(S)-对甲苯亚磺酸酯
(1R,2S,5R)-(-)-薄荷醇(S)-对甲苯亚磺酸酯
(1R,2S,5R)-(-)-薄荷基 (S)-P-甲苯亚磺酸酯
(1R,2S,5R)-(-)-薄荷醇 (S)-对甲苯亚磺酸酯 (-)-(1R)-薄荷基 (S)-对甲苯亚磺酸酯 (S)-(-)-薄荷基 对甲苯亚磺酸酯
(S)-(-)-MENTHYL P-TOLUENESULFINATE
Menthyl (S)-4-methylbenzenesulfinate
(?-(1R)-Menthyl (S)-p-toluenesulfinate
(-)-(1R)-MENTHYL (S)-TOLUENE-4-SULFINATE
(-)-Menthyl (S)-4-methylbenzenesulfinate
(1R,2S,5R)-MENTHYL (S)-P-TOLUENESULFINATE
(1R,2S,5R)-(-)-MENTHYL (S)-P-TOLUENESULFITE
(1R,2S,5R)-(-)-MENTHYL (S)-P-TOLUENESULFINATE
(S)-(-)-P-TOLUENESULFINIC ACID L-MENTHYL ESTER
物理化学性质
制备方法
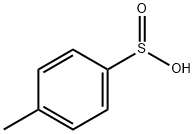
536-57-2
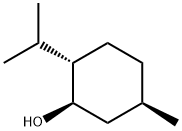
2216-51-5

1517-82-4
以对甲苯亚磺酸和L-薄荷醇为原料合成(S)-((1R,2S,5R)-2-异丙基-5-甲基环己基4-甲基苯亚磺酸酯的一般步骤如下:向一个250 mL经过火焰干燥并用N2吹扫的圆底烧瓶中,依次加入对甲苯亚磺酸(0.525 g,3.36 mmol)、4-溴苄醇(0.598 g,3.20 mmol)和DMAP(0.078 g,0.64 mmol),然后将其溶解于无水CH2Cl2(14 mL)中。向此溶液中一次性加入1-乙基-3-(3-二甲基氨基丙基)碳二亚胺盐酸盐(0.644 g,3.36 mmol),并在室温下搅拌反应混合物16小时。反应完成后,用CH2Cl2(30 mL)稀释反应混合物,依次用1M HCl(30 mL)和盐水(30 mL)洗涤。分离有机层,用MgSO4干燥,过滤后减压除去溶剂。最后,通过快速柱色谱法纯化粗产物。
参考文献:
[1] Synthesis (Germany), 2018, vol. 50, # 24, p. 4855 - 4866
[2] Chemical and Pharmaceutical Bulletin, 1982, vol. 30, # 5, p. 1646 - 1652
[3] Chemical and Pharmaceutical Bulletin, 1982, vol. 30, # 5, p. 1646 - 1652
[4] Molecular Crystals and Liquid Crystals Science and Technology, Section A: Molecular Crystals and Liquid Crystals, 2001, vol. 356, p. 371 - 387
[5] Tetrahedron, 1999, vol. 55, # 8, p. 2311 - 2316
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | M1044 | (1R,2S,5R)-(-)-薄荷基(S)-对甲苯亚磺酸酯 (1R,2S,5R)-(-)-Menthyl (S)-p-Toluenesulfinate | 1517-82-4 | 5G | 230元 |
| 2025/12/22 | M1044 | (1R,2S,5R)-(-)-薄荷基(S)-对甲苯亚磺酸酯 (1R,2S,5R)-(-)-Menthyl (S)-p-Toluenesulfinate | 1517-82-4 | 25G | 940元 |
| 2025/09/19 | 42811 | (1R,2S,5R)-(-)-薄荷基(S)-对甲苯亚磺酸酯 (1R,2S,5R)-(-)-Menthyl (S)-p-toluenesulfinate, 98%, Thermo Scientific Chemicals | 1517-82-4 | 10g | 2074元 |
