21461-84-7
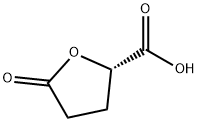 21461-84-7 结构式
21461-84-7 结构式
基本信息
5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID
(S)-(+)-5-OXO-2-TETRAHYDROFURANCARBOXYLIC ACID
(S)-(+)-5-OXO-2-TETRAHYDROFUROIC ACID
(S)-(+)-5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID
(S)-5-OXOTETRAHYDROFURAN-2-CARBOXYLIC ACID
(S)-(+)-GAMMA-CARBOXY-GAMMA-BUTYROLACTONE
(S)-GAMMA-CARBOXY-GAMMA-BUTYROLACTONE
(S)-(+)-TETRAHYDRO-5-OXO-2-FURANCARBOXYLIC ACID
(S)-(+)-TETRAHYDRO-5-OXO-2-FURANECARBOXYLIC ACID
TIMTEC-BB SBB006581
TOF
(S)-(+)-5-OXO-2-TETRAHYDROFURANCARBOXYLI C ACID, 98% (99% EE/GLC)
(S)-5-Oxotetrahydro-2-furancarboxylic acid
2-Furancarboxylic acid, tetrahydro-5-oxo-, (2S)-
(s)-γ-carboxy-γ-butyrolactone
(2S)-5-OXO-2-TETRAHYDROFURANCARBOXYLIC ACID
(S)-γ-Carboxy-γ-butyrolactone, (S)-5-Oxotetrahydro-2-furancarboxylic acid
(S)-(+)-5-OXO-2-TETRAHYDROFURANCARBOXILIC ACID, 98%
(2S)-5-Oxotetrahydrofuran-2-carboxylic acid
物理化学性质
制备方法
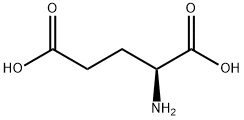
56-86-0

21461-84-7
以L-谷氨酸为原料合成(S)-5-氧代-2-四氢呋喃羧酸的一般步骤:将L-谷氨酸(10.07g,0.068mol,JK CHEMICA)溶解于20mL水中,置于锥形瓶中。在-5℃条件下,向该溶液中缓慢加入NaNO2(7.0g,0.102mol,汕头西龙化学工厂)在20mL水中的溶液,同时加入HCl和40mL水。反应混合物在室温下持续搅拌12小时。随后,将反应混合物在低于50℃的条件下进行真空蒸发,得到黄色油状物。将此油状物溶解于EtOAc中,过滤去除形成的固体,并用EtOAc洗涤固体。合并滤液与洗涤液,用Na2SO4干燥。最后,通过真空浓缩去除溶剂,得到(S)-5-氧代-2-四氢呋喃羧酸,为浅黄色油状物(8.1g,产率91.6%)。产物经质谱(ESI,阳离子模式)分析,m/z:130.9(M+1);1H NMR(400MHz,CDCl3)数据如下:δ2.27-2.41(m,1H),2.44-2.65(m,3H),5.09(m,1H),9.12-9.55(m,1H)。
参考文献:
[1] Journal of Organic Chemistry, 2010, vol. 75, # 2, p. 390 - 398
[2] Patent: WO2010/45095, 2010, A1. Location in patent: Page/Page column 69; 70
[3] Synthesis, 2007, # 15, p. 2279 - 2286
[4] Tetrahedron, 2009, vol. 65, # 48, p. 9997 - 10001
[5] Carbohydrate Research, 1983, vol. 124, p. 35 - 42
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW02434484705 | 5-氧四氢呋喃-2-羧酸 5-Oxotetrahydrofuran-2-carboxylic acid | 21461-84-7 | 100g | 840元 |
| 2025/12/22 | XW02434484704 | 5-氧四氢呋喃-2-羧酸 5-Oxotetrahydrofuran-2-carboxylic acid | 21461-84-7 | 25g | 248元 |
| 2025/12/22 | XW02434484703 | 5-氧四氢呋喃-2-羧酸 5-Oxotetrahydrofuran-2-carboxylic acid | 21461-84-7 | 10g | 109元 |
