228244-04-0
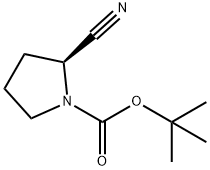 228244-04-0 结构式
228244-04-0 结构式
基本信息
2-氰基吡咯烷-1-甲酸叔丁酯
(S)-1-BOC-2-氰基吡咯烷
(S)-1-N-Boc-2-吡咯烷甲腈
(S)-2-氰基吡咯烷-1-甲酸叔丁酯
1-N-BOC-2-CYANO-PYRROLIDINE
1-n-boc-2-pyrrolidinonitrile
2-CYANO-PYRROLIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
2-CYANOPYRROLIDINE, N-BOC PROTECTED
TERT-BUTYL 2-CYANOPYRROLIDINE-1-CARBOXYLATE
(s)- N-boc-2-cyano-piperidine
(S)-1-(tert-Butoxycarbonyl)-2-Pyrrolidinecarbonitrile
(S)-1-Boc-2-cyanopyrrolidine
(S)-1-BOC-2-Pyrrolidinecarbonitrile
(S)-1-Boc-2-cyanopiperidine
物理化学性质
安全数据
制备方法
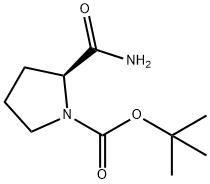
35150-07-3

228244-04-0
通用方法:将N-叔丁氧羰基-L-脯氨酰胺(5g,23.3mmol)和氰尿酰氯(2.58g,14.0mmol)溶于DMF(10mL)中,室温搅拌反应1小时(反应进程通过TLC监测)。反应完成后,将反应混合物用EtOAc萃取,有机相依次用水和饱和食盐水洗涤,无水Na2SO4干燥,减压浓缩。粗产物通过硅胶柱色谱纯化,洗脱剂为石油醚/乙酸乙酯(1/1,v/v),得到(S)-1-N-Boc-2-吡咯烷甲腈(3.48g,收率76%)为白色固体。1H NMR(300MHz,CDCl3)δ4.76(s,1H),3.51(s,2H),2.34-2.31(m,1H),2.17-2.10(m,2H),1.90-1.87(m,1H),1.49(s,9H)。MS(ESI)m/z 197 [M + H]+。
参考文献:
[1] Journal of Agricultural and Food Chemistry, 2012, vol. 60, # 35, p. 8544 - 8551
[2] Canadian Journal of Chemistry, 2007, vol. 85, # 2, p. 85 - 95
[3] Synthetic Communications, 2009, vol. 39, # 3, p. 395 - 406
[4] Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 23, p. 7418 - 7429
[5] European Journal of Medicinal Chemistry, 2014, vol. 75, p. 111 - 122
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0224245944503 | (S)-1-Boc-2-氰基哌 (S)-1-Boc-2-Cyanopiperidine | 228244-04-0 | 1g | 870元 |
| 2025/12/22 | XW0224245944502 | (S)-1-Boc-2-氰基哌 (S)-1-Boc-2-Cyanopiperidine | 228244-04-0 | 250mg | 227元 |
| 2025/12/22 | XW0224245944501 | (S)-1-Boc-2-氰基哌 (S)-1-Boc-2-Cyanopiperidine | 228244-04-0 | 100mg | 113元 |

