140695-84-7
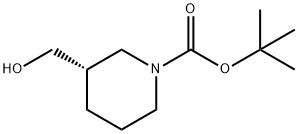 140695-84-7 结构式
140695-84-7 结构式
基本信息
(S)-1-叔丁氧羰基-3-羟甲基哌啶
(S)-1-N-BOC-3-羟甲基哌啶
N-BOC-(3S)-PIP(3-CH2OH)
(S)-1-BOC-3-(HYDROXYMETHYL)PIPERIDINE
(S)-1-N-BOC-3-HYDROXYMETHYL-PIPERIDINE
(S)-3-HYDROXYMETHYL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
(S)-N-(TERT-BUTOXYCARBONYL)-3-HYDROXYMETHYLPIPERIDINE
(S)-TERT-BUTYL 3-(HYDROXYMETHYL)PIPERIDINE-1-CARBOXYLATE
(S)-TERT-BUTYL 3-(HYDROXYMETHYL)TETRAHYDRO-1(2H)-PYRIDINECARBOXYLATE
(S)-N-Boc-3-piperidinemethanol
(S)-1-BOC-3-(HYROXYMETHYL)PIPERIDINE
(S)-3-(Hydroxymethyl)piperidine, N1-BOC protected
(S)-N-Boc-3-(hyroxymethyl)piperidine
物理化学性质
制备方法
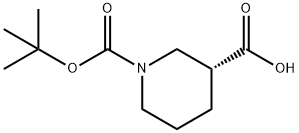
163438-09-3
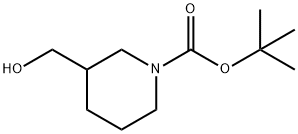
116574-71-1
以 (R)-1-(叔丁氧羰基)哌啶-3-羧酸为原料合成 3-(羟甲基)哌啶-1-甲酸叔丁酯的一般步骤如下: 实施例58:N-(2-甲基丙基)-N-(3,5-二氯苯基甲基)-(3R)-哌啶-3-基甲胺1.5L-酒石酸盐的合成: (i)在室温下,将硼烷-四氢呋喃络合物(1M的THF溶液,65.4ml,65.4mmol,3当量)缓慢滴加到 (R)-N-Boc-哌啶-3-羧酸(5g,21.8mmol,1当量)的THF(50ml)溶液中。反应混合物在室温下搅拌16小时。随后,将溶液冷却至0℃,并小心加入2N氢氧化钠水溶液(250ml)进行水解。水解后的混合物加热回流48小时。反应完成后,将混合物再次冷却至0℃,并用乙醚(3×100ml)萃取。合并有机层,用盐水(100ml)洗涤,硫酸镁干燥,过滤并真空浓缩。残余物通过快速色谱法纯化,使用50-70%乙酸乙酯的异己烷溶液作为洗脱剂,得到标题化合物 (3R)-3-(羟甲基)哌啶-1-羧酸叔丁酯(4.56g,收率98%)。 1H NMR(300MHz,CDCl3)δ:4.0-2.7(6H,br m),2.17-1.19(5H,br m),1.46(9H,s)。
参考文献:
[1] Patent: WO2005/118531, 2005, A1. Location in patent: Page/Page column 74
[2] Patent: WO2006/12308, 2006, A1. Location in patent: Page/Page column 68
[3] Patent: WO2017/103611, 2017, A1. Location in patent: Paragraph 00298
