25173-72-2
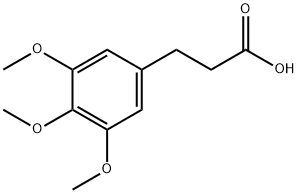 25173-72-2 结构式
25173-72-2 结构式
基本信息
3-(3,4,5-三甲氧基苯基)丙酸, 98+%
3-(3,4,5-TRIMETHOXYPHENYL)PROPIONIC ACID
3,4,5-TRIMETHOXYHYDROCINNAMIC ACID
AKOS BBS-00006959
B-(3,4,5-TRIMETHOXYPHENYL)PROPIONIC ACID
BETA-(3,4,5-TRIMETHOXYPHENYL)PROPIONIC ACID
LABOTEST-BB LT00233171
TIMTEC-BB SBB000794
3,4,5-Trimethoxyphenylpropionic acid
Benzenepropanoic acid, 3,4,5-trimethoxy-
3-(3,4,5-TRIMETHOXYPHENYL)PROPIONIC ACID , 98+%
3,4,5-Trimethoxybenzenepropanoic acid
3,4,5-Trimethoxybenzenepropionic acid
物理化学性质
制备方法
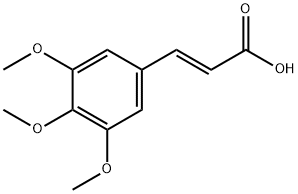
20329-98-0

25173-72-2
以(E)-3-(3,4,5-三甲氧基苯基)丙烯酸为原料合成3-(3,4,5-三甲氧基苯基)丙酸的一般步骤(通过微波辐射方法):在100 mL Erlenmeyer烧瓶中,将3,4,5-三甲氧基肉桂酸(0.72 g,0.003 mol)和PdCl2(55 mg,0.31 mmol)悬浮于10%氢氧化钠溶液(6-10 mL)中,随后分批加入甲酸(8-12 mL)。将混合物在微波辐射下反应3-5分钟,直至原料完全消失。反应完成后,将冷却的混合物倒入冰水中,用5% HCl酸化,并用二氯甲烷(3×10 mL)萃取。有机层用水洗涤后,用无水Na2SO4干燥。蒸发去除溶剂,粗产物通过乙酸乙酯和己烷混合溶剂重结晶,得到白色固体3-(3,4,5-三甲氧基苯基)丙酸,产率84%,熔点102℃(文献值101-102℃)。1H NMR(CDCl3)δ:6.70(2H,s,H-2和H-6),3.84(9H,s,3-OCH3,4-OCH3和5-OCH3),2.92(2H,t,Ar-CH2-CH2-COOH),2.70(2H,t,-CH2-CH2-COOH);13C NMR δ:178.1(COOH),153.1(C-3和C-5),135.87(C-4和C-1),105.3(C-2和C-6),60.8(4-OCH3),56.1(3-OCH3和5-OCH3),35.5(Ar-CH2-),31.0(-CH2-COOH)。
参考文献:
[1] Journal of Organic Chemistry, 2015, vol. 80, # 24, p. 11953 - 11962
[2] Chemistry Letters, 2003, vol. 32, # 12, p. 1186 - 1187
[3] Patent: US2004/118673, 2004, A1. Location in patent: Page 7
[4] Bioorganic Chemistry, 2018, vol. 80, p. 408 - 421
[5] Angewandte Chemie - International Edition, 2014, vol. 53, # 30, p. 7785 - 7788
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-W022390 | 3-(3,4,5-三甲氧基苯基)丙酸 3-(3,4,5-Trimethoxyphenyl)propanoic acid | 25173-72-2 | 1g | 100元 |
| 2025/12/22 | HY-W022390 | 3-(3,4,5-三甲氧基苯基)丙酸 3-(3,4,5-Trimethoxyphenyl)propanoic acid | 25173-72-2 | 10 mM * 1 mLin DMSO | 110元 |
| 2025/12/22 | HY-W022390 | 3-(3,4,5-三甲氧基苯基)丙酸 3-(3,4,5-Trimethoxyphenyl)propanoic acid | 25173-72-2 | 5 g | 250元 |
