33301-41-6
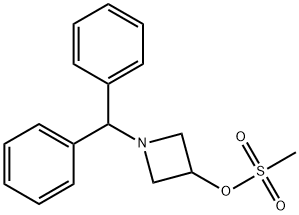 33301-41-6 结构式
33301-41-6 结构式
基本信息
1-二苯甲基氮杂环丁-3-基甲磺酸酯
1-BENZHYDRYL-3-METHANESULFONYLOXY AZETIDINE
1-BENZHYDRYLAZETIDIN-3-YL METHANESULFONATE
1-DIPHENYLMETHYL-3-AZETIDINYL METHANESULFONATE
1-(DIPHENYLMETHYL)-3-(METHANESULFONYLOXY)AZETIDINE
BUTTPARK 37\04-14
1-(diphenylmethyl)-3-(methanesulfonyloxy)azetinide
1-Diphenylmethyl-3-azetidinyl Methanethiosulfonate
1-Benzhydryl-3-methanesulphonyloxyazetidine
1-BENZHYDRYL-3-METHANESULFONATOAZETIDINE 98%
Methanesulfonic acid 1-benzhydryl-azetidin-3-yl ester
1-(Diphenylmethyl)-3-(mesyloxy)azetidine
1-(Diphenylmethyl)-3-[(methylsulfonyl)oxy]azetidine
物理化学性质
制备方法
18621-17-5

124-63-0

33301-41-6
在氮气保护下,将1-二苯甲基-3-羟基氮杂环丁烷(15.0 g,62.7 mmol)溶解于无水二氯甲烷(1 mL)中,冷却至0℃(冰水浴)。向此溶液中加入干燥的三乙胺(25 mL,94.0 mmol)。随后,通过加压均流加料漏斗缓慢滴加甲基磺酰氯(5.8 mL,75.2 mmol)的无水二氯甲烷(50 mL)溶液。滴加完毕后,移除冷却浴,继续搅拌反应混合物2小时。反应完成后,用去离子水(70 mL)淬灭反应,分离有机层和水层。水层用二氯甲烷(2×100 mL)萃取。合并有机层,用无水硫酸镁干燥,过滤后浓缩,得到澄清无色油状物。向此油状物中加入己烷(100 mL),促使产物固化。通过真空过滤收集固体产物,并在高真空下干燥,得到19.7 g(收率100%)的1-二苯甲基-3-甲烷磺酸氮杂环丁烷(12),为无色固体。
参考文献:
[1] Patent: US2005/54697, 2005, A1. Location in patent: Page/Page column 78
[2] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 4, p. 1084 - 1088
[3] Patent: WO2011/61214, 2011, A1. Location in patent: Page/Page column 85-86
[4] Patent: US2014/288045, 2014, A1. Location in patent: Paragraph 0605
[5] Patent: WO2016/44772, 2016, A1. Location in patent: Paragraph 319
