344331-90-4
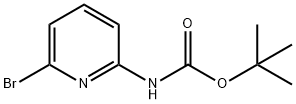 344331-90-4 结构式
344331-90-4 结构式
基本信息
2-(BOC-氨基)-6-溴吡啶
(6-溴吡啶-2-基)氨基甲酸叔丁酯
6-溴-2-吡啶-氨基甲酸-1,1-二甲基乙基酯
2-(BOC-AMino)-6-broMopyridine
N-Boc-2-AMino-6-broMopyridine
tert-Butyl 6-bromopyridin-2-ylcarbamate
t-butyl N-(6-bromo-2-pyridinyl)carbamate
6-BROMO-2-TERT-BUTOXYCARBONYLAMINO-PYRIDINE
tert-butyl N-(6-bromopyridin-2-yl)carbamate
Benzenemethanesulfonylchloride,9-(trifluoromethyl)-
(6-BroMo-pyridin-2-yl)-carbaMic acid tert-butyl ester
(6-BROMO-2-PYRIDINYL)-CARBAMIC ACID,1,1-DIMETHYLETHYL ESTER
物理化学性质
制备方法
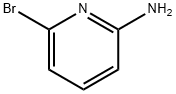
19798-81-3

24424-99-5

344331-90-4
步骤1. 合成6-溴-2-叔丁氧羰基氨基吡啶: 向含有6-溴吡啶-2-胺(3g, 17.34mmol)、三乙胺(3.14mL, 22.54mmol)和DMAP(0.424g, 3.47mmol)的二氯甲烷(24mL)溶液中,缓慢加入溶解有二碳酸二叔丁酯(4.83mL, 20.81mmol)的二氯甲烷(6mL)溶液。反应混合物在室温下搅拌约24小时。反应完成后,用水、饱和食盐水和乙酸乙酯稀释反应混合物。分离水层,并用乙酸乙酯萃取。合并有机层,用无水硫酸钠干燥,减压浓缩。通过柱色谱法纯化残余物,得到6-溴-2-叔丁氧羰基氨基吡啶,为白色固体。产量: 1.67g。LCMS (m/z): 274.9 [M+H]+; 保留时间: 0.54分钟(主要峰), 0.95分钟。
参考文献:
[1] Patent: WO2012/101066, 2012, A1. Location in patent: Page/Page column 77
[2] Journal of Medicinal Chemistry, 2010, vol. 53, # 24, p. 8709 - 8715
[3] Patent: WO2017/205536, 2017, A2. Location in patent: Page/Page column 193; 194
[4] Patent: WO2004/113331, 2004, A1. Location in patent: Page 68
[5] Patent: WO2006/53791, 2006, A2. Location in patent: Page/Page column 39-40
