36276-24-1
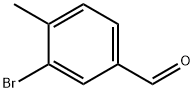 36276-24-1 结构式
36276-24-1 结构式
基本信息
3-bromo-4-methyl Benzalehyde
benzaldehyde, 3-bromo-4-methyl-
3-Bromo-4-methylbenzaldehyde 97%
JR-13806, 3-Bromo-4-methylbenzaldehyde, 97%
3-bromo-4-methylbenzaldehyde(SALTDATA: FREE)
物理化学性质
制备方法
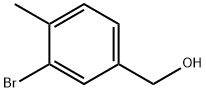
68120-35-4

36276-24-1
以3-溴-4-甲基苄醇为原料合成3-溴-4-甲基苯甲醛的一般步骤:向3-溴-4-甲基苄醇(4.5g,22.4mmol)的氯仿(100mL)溶液中加入二氧化锰(15g,172mmol)。将反应混合物在搅拌下于70℃的油浴中回流18小时。反应完成后,将混合物通过硅藻土过滤,并真空除去溶剂,得到粗产物。粗产物通过柱色谱(150mL SiO2,洗脱剂为乙酸乙酯与己烷的混合液,体积比为1:9)纯化,得到3-溴-4-甲基苯甲醛(3.2974g,产率74%),为白色结晶固体。产物表征数据如下:1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 8.02 (d, J = 1.6 Hz, 1H), 7.70 (dd, J = 7.6, 1.6 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 2.47 (s, 3H); 13C NMR (100.6 MHz, CDCl3) δ 190.4, 145.1, 135.8, 133.4, 131.3, 128.3, 125.6, 23.4; GC-MS (M)+ C8H7OBr计算值197.9680,实测值197.9665。
参考文献:
[1] Patent: WO2013/40227, 2013, A2. Location in patent: Page/Page column 37; 38
[2] Journal of Medicinal Chemistry, 2013, vol. 56, # 21, p. 8432 - 8454
[3] Journal of Medicinal Chemistry, 2010, vol. 53, # 10, p. 3973 - 4001
[4] Patent: US2003/195259, 2003, A1
