367-24-8
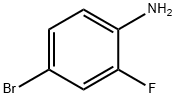 367-24-8 结构式
367-24-8 结构式
基本信息
2-氟-4-溴苯胺
4-溴-2-氟苯胺,98+%
4-溴邻氟苯胺
4-BROMO-2-FLUORO-PHENYLAMINE
AKOS BBS-00003609
Benzenamine, 4-bromo-2-fluoro-
2-Fluoro-4-Bromoaniline
Bromofluoroaniline4
-BROMO-2-FLUOROANILINE, 98%
4-Bromo-2-fluoroaniline,98+%
4-Bromo-2-fluoroaniline 99%
4-Bromo-2-fluoroaniline99%
4-bromo-2-fluoro-Benzenamine
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
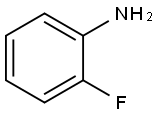
348-54-9

367-24-8
以邻氟苯胺为起始原料合成4-溴-2-氟苯胺的一般步骤如下:参照文献方法,将2-氟苯胺(12)(2.22 g,20 mmol)悬浮于CHCl3(50 mL)中,加入N-溴代琥珀酰亚胺(NBS)(3.56 g,20 mmol)。将反应混合物在0℃下搅拌2小时。反应进程通过薄层色谱(TLC)监测。反应完成后,用饱和NaHCO3水溶液淬灭反应,随后加入Na2S2O3水溶液。用CH2Cl2(3×20 mL)萃取混合物。合并有机层,用MgSO4干燥,随后在真空下浓缩。粗产物通过快速柱色谱法(洗脱剂:己烷/乙酸乙酯,10:1)纯化,得到目标产物13,为橙色油状物(3.42 g,收率90%)。TLC(己烷/乙酸乙酯,6:1)显示Rf值为0.35。1H NMR(300 MHz,CDCl3)δ:7.14(1H,dd,J = 2.1, 10.5 Hz),7.05(1H,d,J = 8.4 Hz),6.65(1H,t,J = 9.0 Hz),3.72(2H,br s)。13C NMR(75 MHz,CDCl3)δ:151.5(d,JCF = 241.5 Hz),133.9(d,JCF = 12.8 Hz),127.5(d,JCF = 3.0 Hz),118.8(d,JCF = 21.8 Hz),117.9(d,JCF = 3.8 Hz),109.0(d,JCF = 9.0 Hz)。所得化合物的光谱数据与文献报道一致。
参考文献:
[1] Journal of Organic Chemistry, 2005, vol. 70, # 11, p. 4267 - 4271
[2] Beilstein Journal of Organic Chemistry, 2012, vol. 8, p. 744 - 748
[3] Tetrahedron Letters, 2015, vol. 56, # 41, p. 5646 - 5650
[4] Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 22, p. 5852 - 5869
[5] Patent: CN106905168, 2017, A. Location in patent: Paragraph 0026-0029
知名试剂公司产品信息
4-Bromo-2-fluoroaniline, 99%(367-24-8)
