37813-30-2
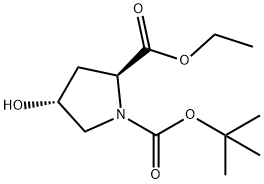 37813-30-2 结构式
37813-30-2 结构式
基本信息
1-叔丁氧羰基-4-羟基-L-脯氨酸乙酯
1-叔丁氧羰基-4R-羟基-L-脯氨酸乙酯
1-叔丁氧羰基-(4R)-羟基-2-脯氨酸乙酯
1-叔丁氧羰基-(4R)-羟基-2-脯氨酸乙脂
(2S,4R)-1-BOC-2-甲酸乙酯-4-羟基吡咯烷
Boc-L-Hyp-oet
Boc-L-Hydroxy-Proline Ethyl Ester
1-Boc-L-hydroxyproline ethyl ester
1-Boc-4-Hydroxy-L-Proline Ethyl Ester
(Tert-Butoxy)Carbonyl Hyp-OEt (Syrup)
(4R)-1-Boc-4-hydroxy-L-proline ethyl ester
1-tert-butoxycarbonyl-4-hydroxy-L-proline ethyl ester
1-tert-Butoxycarbonyl-4R-hydroxy-L-proline ethyl ester
1-tert-butoxycarbonyl-4-hydroxy-L-proline ethyl ester USP/EP/BP
物理化学性质
制备方法

24424-99-5
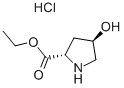
33996-30-4

37813-30-2
步骤2:将(2S,4R)-4-羟基吡咯烷-2-甲酸乙酯盐酸盐(146.2 g,0.747 mol)溶解于二氯甲烷(1600 mL)和乙醇(100 mL)的混合溶剂中,冷却至0℃。在搅拌下,向该溶液中加入三乙胺(113.4 g,1.12 mol,156.2 mL)。随后,分批加入二碳酸二叔丁酯(195.6 g,0.90 mol)。反应混合物在0℃下搅拌15分钟,然后升至室温继续搅拌16小时。反应完成后,将混合物浓缩至约800 mL,用水洗涤。有机层用硫酸镁干燥,过滤后浓缩。通过硅胶柱色谱法(洗脱剂:20%乙酸乙酯-二氯甲烷)纯化,得到Boc-L-羟脯氨酸乙酯(193.7 g,100%)为黄色油状物。质谱(M + Na):m/e 282。1H-NMR(CDCl3)δ 1.30(t,3H),1.45(s,9H),1.75(m,1H),2.10(m,1H),2.30(m,1H),3.45和3.55(d,1H,两个旋转异构体),3.65(dd,1H),4.25(m,2H),4.40和4.45(t,1H,两个旋转异构体),4.55(宽s,1H)。
参考文献:
[1] Patent: WO2008/8327, 2008, A2. Location in patent: Page/Page column 15-16
[2] Patent: US2011/3780, 2011, A1. Location in patent: Page/Page column 4
[3] Journal of Heterocyclic Chemistry, 2011, vol. 48, # 5, p. 1132 - 1139
[4] Patent: TW2017/8221, 2017, A. Location in patent: Page/Page column 38
[5] Synlett, 2011, # 14, p. 2059 - 2063