39827-11-7
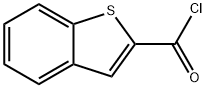 39827-11-7 结构式
39827-11-7 结构式
基本信息
BENZO[B]THIOPHENE-2-CARBONYL CHLORIDE
BUTTPARK 98\04-18
1-Benzothiophene-2-carbonyl chloride
Thianaphthene-2-carbonyl chloride
Benzothiophene-2-Carbonyl Chloride
Benzo[b]thiophene-2-carbonyl chloride (6CI, 7CI, 9CI)
Benzobüthiophene-2-carbonyl chloride, 98%
1-Benzothiophene 2-carboxylic acid chloride
Benzo[b]thiophene-2-carboxylic acid chloride
物理化学性质
安全数据
制备方法
6314-28-9
![苯并[B]噻吩-2-羰酰氯](/CAS/GIF/39827-11-7.gif)
39827-11-7
以苯并[b]噻吩-2-羧酸为原料合成苯并[b]噻吩-2-碳酰氯的一般步骤如下:将苯并[b]噻吩-2-羧酸(356.42 mg,2 mmol)悬浮于无水甲苯(6 mL)中,加入亚硫酰氯(4.4 mL,60 mmol)和N,N-二甲基甲酰胺(DMF,0.05 mL)。在室温下搅拌后,将混合物加热回流8小时。反应完成后,在减压下除去挥发物,得到苯并[b]噻吩-2-碳酰氯,为黄色粉末。通过硅胶快速色谱法纯化,使用乙酸乙酯/己烷(1:9,v/v)作为洗脱剂,得到目标产物(393.64 mg,收率94.9%),为白色粉末。产物的光谱数据与文献报道一致。1H NMR (300 MHz, CDCl3): δ 8.31 (s, 1H), 7.04-7.89 (m, 2H), 7.60-7.46 (m, 2H); 13C NMR (300 MHz, CDCl3): δ 161.14, 144.07, 138.05, 136.59, 135.89, 128.75, 126.68, 125.66, 122.91。
参考文献:
[1] Journal of the American Chemical Society, 2011, vol. 133, # 11, p. 3764 - 3767
[2] Patent: WO2012/99785, 2012, A2. Location in patent: Page/Page column 25
[3] Tetrahedron Asymmetry, 2003, vol. 14, # 3, p. 339 - 346
[4] Organometallics, 2015, vol. 34, # 12, p. 3065 - 3071
[5] Journal of the American Chemical Society, 2005, vol. 127, # 43, p. 15010 - 15011
