405224-23-9
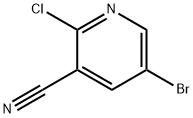 405224-23-9 结构式
405224-23-9 结构式
基本信息
5-溴-2-氯烟腈
5-溴-2-氯吡啶-3-腈
2-氯-5-溴吡啶-3-甲腈
5-溴-2-氯-3-氰基吡啶
5-Bromo-2-chloronicotinon...
5-Bromo-2-chloronicotinonitrile
5-Bromo-2-chloro-3-cyanopyridine
2,5-dichloropyridine-3-carbonitrile
5-Bromo-2-chloropyridine-3-carbonitrile
5-Bromo-2-chloro-3-pyridinecarbonitrile
3-Pyridinecarbonitrile, 5-broMo-2-chloro-
5-Bromo-2-chloro-3-cyanopyridine ISO 9001:2015 REACH
5-Bromo-2-chloropyridine-3-carbonitrile, 5-Bromo-2-chloro-3-cyanopyridine
物理化学性质
安全数据
toxic compounds or compounds which causing chronic effects
Eye Dam. 1
Skin Irrit. 2
STOT SE 3
制备方法
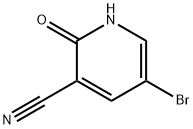
405224-22-8

405224-23-9
5-溴-2-氯烟腈(13)的合成:将5-溴-2-氧代-1,2-二氢吡啶-3-甲腈(12)(69mg,0.35mmol)与五氯化磷(PCl5)(109mg,0.52mmol)在三氯氧磷(POCl3)(5mL)中混合,回流反应过夜。反应完成后,将反应混合物冷却至室温,并缓慢滴加到冰水中。用4M氢氧化钠(NaOH)溶液调节pH至8,然后用二氯甲烷(CH2Cl2)(3×30mL)进行萃取。合并有机相,依次用碳酸氢钠(NaHCO3)水溶液和饱和食盐水洗涤,无水硫酸钠(Na2SO4)干燥,减压浓缩。通过柱色谱法(石油醚/乙酸乙酯=20:1)纯化,得到目标产物5-溴-2-氯烟腈(13)(63mg,收率84%),为黄色固体。熔点:138~139℃。1H NMR(400MHz,CDCl3):δ8.66(d,J=2.4Hz,1H),8.12(d,J=2.4Hz,1H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry Letters, 2003, vol. 13, # 9, p. 1577 - 1580
[2] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 20, p. 5578 - 5585
[3] Bioorganic and Medicinal Chemistry Letters, 2015, vol. 25, # 16, p. 3251 - 3255
[4] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 23, p. 6578 - 6581
[5] Patent: US2004/19052, 2004, A1. Location in patent: Page/Page column 17

