406463-06-7
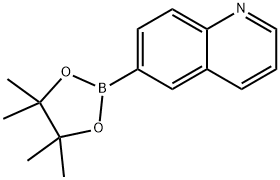 406463-06-7 结构式
406463-06-7 结构式
基本信息
6-喹啉硼酸匹那醇酯
6-喹啉硼酸频那醇酯
6-喹啉硼酸频呢醇酯
6-喹啉频哪醇硼酸酯
喹啉-6-硼酸频那醇酯
喹啉-6-硼酸频哪醇酯
6-喹啉基硼酸频哪醇酯
6-(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)喹啉
6-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)喹啉
Quinoline-6-boronic acid,pinacol ester
6-Quinolineboronic acid pinacol ester 97%
6-Quinolineboronic acid pinacol ester, 97%
2-(Quinolin-6-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Quinoline, 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline, 6-Quinolylboronic acid pinacol ester
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
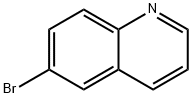
5332-25-2

73183-34-3

406463-06-7
步骤2. 6-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)喹啉的合成 在氮气保护下,向一个预先干燥并吹扫的100 mL圆底烧瓶中加入6-溴喹啉(5 g,23.92 mmol,1.00当量)的四氢呋喃(20 mL)溶液。随后依次加入乙酸钾(KOAc,3.55 g,1.50当量)、联硼酸频那醇酯(18.23 g,71.77 mmol,3.00当量)和[1,1'-双(二苯基膦)二茂铁]二氯化钯(II)(Pd(dppf)2Cl2,1.95 g)。将反应混合物置于80℃油浴中搅拌反应过夜。反应完成后,过滤除去不溶性固体。滤液经减压浓缩后,通过硅胶柱色谱纯化,洗脱剂为乙酸乙酯/石油醚(1:50,v/v)。最终得到6.62 g(产率109%)的6-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)喹啉,为红色油状物。
参考文献:
[1] Patent: US2012/277224, 2012, A1. Location in patent: Page/Page column 90
[2] Patent: WO2008/51493, 2008, A2. Location in patent: Page/Page column 104
[3] Patent: CN105254613, 2016, A. Location in patent: Paragraph 0071; 0072; 0073
[4] Patent: WO2015/162538, 2015, A1. Location in patent: Page/Page column 66
[5] Organic Letters, 2014, vol. 16, # 17, p. 4562 - 4565
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | T3507 | 6-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)喹啉 6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline | 406463-06-7 | 1g | 120元 |
| 2025/09/19 | 43964 | 6-喹啉硼酸频那醇酯 1GR 6-Quinolineboronic acid pinacol ester, 97%, Thermo Scientific Chemicals | 406463-06-7 | 5g | 3870元 |
| 2025/05/22 | 43964 | 6-喹啉硼酸频那醇酯 1GR 6-Quinolineboronic acid pinacol ester, 97%, Thermo Scientific Chemicals | 406463-06-7 | 1g | 1000元 |
