433226-06-3
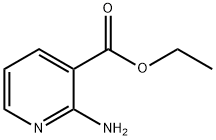
 433226-06-3 结构式
433226-06-3 结构式
基本信息
Ethyl 2-amino-5-bromopyridine-3-carboxylate
3-Pyridinecarboxylic acid, 2-aMino-5-broMo-, ethyl ester
Ethyl 2-amino-5-bromopyridine-3-carboxylate, 2-Amino-5-bromo-3-(ethoxycarbonyl)pyridine
物理化学性质
制备方法
13362-26-0

433226-06-3
以2-氨基烟酸乙酯为原料合成2-氨基-5-溴烟酸乙酯的一般步骤:在0℃下,向搅拌的2-氨基烟酸乙酯(15g; 90.36mmol; 1当量)的无水THF(150mL)溶液中分批加入N-溴代琥珀酰亚胺(NBS)(16g; 90.36mmol; 1当量)。将得到的混合物在23℃下搅拌18小时。反应完成后,将混合物倒入冰冷的饱和NaHCO3水溶液中,用乙酸乙酯(3×200mL)萃取有机组分。合并的有机层用盐水溶液洗涤,用无水硫酸钠干燥,过滤并减压浓缩至干,得到2-氨基-5-溴烟酸乙酯(22g,100%),为灰白色固体。产物经1H NMR(DMSO-d6)确认:δ 8.29(d, 1H, J = 3Hz),8.12(d, 1H, J = 2Hz),7.31(s, 2H),4.29(q, 2H, J = 7Hz),1.30(t, 3H, J = 7Hz)。LCMS分析结果:m/z = 245.0 [M + H]+, 247.0 [M + 2H]+, 保留时间(RT)= 3.34分钟(程序P1,柱W)。
参考文献:
[1] Patent: WO2015/95128, 2015, A1. Location in patent: Paragraph 0239; 0242; 0243
[2] European Journal of Medicinal Chemistry, 2018, vol. 158, p. 236 - 246
[3] Patent: CN104402881, 2016, B. Location in patent: Paragraph 0022; 0023
[4] Patent: WO2007/67416, 2007, A2. Location in patent: Page/Page column 64; 134
