450412-28-9
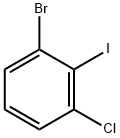 450412-28-9 结构式
450412-28-9 结构式
基本信息
1-溴-2-碘-3-氯苯
1-溴-3-氯-2-碘代苯
1-溴-3-氯-2-碘-苯
1-溴-3-氯-2-碘苯,97%
2-Bromo-6-chloroiodobenzene 99%
1-Bromo-3-chloro-2-Iodobenzenne
Benzene, 1-bromo-3-chloro-2-iodo-
1-Bromo-3-chloro-2-iodobenzene,97%
物理化学性质
制备方法
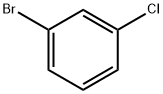
108-37-2

450412-28-9
步骤1. 1-溴-3-氯-2-碘苯的合成:在氮气保护下,向装有二异丙胺(76 mL,0.4 mol)的无水THF(664 mL)溶液中,于-78℃下经1小时缓慢滴加2.5 M正丁基锂(n-BuLi,160 mL,0.4 mol)的正己烷(220 mL)溶液。维持反应温度在-78℃,继续搅拌1小时后,缓慢滴加1-溴-3-氯苯(76 g,0.4 mol)的无水THF(300 mL)溶液。滴加完毕后,保持-78℃反应1小时,随后缓慢滴加碘(101 g,0.4 mol)的无水THF(400 mL)溶液。反应体系在2小时内逐渐升温至室温,并在室温下继续搅拌18小时。反应完成后,将混合物在减压下浓缩,得到粗产物(120 g)。粗产物经减压蒸馏纯化,得到1-溴-3-氯-2-碘苯(115 g,收率91%)。产物结构经1H NMR(400 MHz,CDCl3)和质谱(MS)确认:1H NMR δ 7.12-7.18(t,1H),7.35-7.41(dd,1H),7.49-7.54(dd,1H);MS(E/Z):317(M+H+)。
参考文献:
[1] Patent: WO2008/124575, 2008, A1. Location in patent: Page/Page column 103
[2] Patent: US2010/317697, 2010, A1. Location in patent: Page/Page column 47
[3] Patent: WO2008/124582, 2008, A1. Location in patent: Page/Page column 86-87
[4] Patent: WO2007/117560, 2007, A2. Location in patent: Page/Page column 169
[5] Patent: WO2008/124577, 2008, A1. Location in patent: Page/Page column 69
