454712-26-6
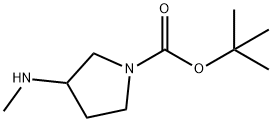 454712-26-6 结构式
454712-26-6 结构式
基本信息
1-Boc-3-甲氨基吡咯烷
N-BOC-3-甲氨基吡咯烷
3-甲氨基吡咯烷-1-甲酸叔丁酯
3-甲氨基-1-叔丁氧羰基吡咯烷
3-(甲氨基)吡咯-1-甲酸叔丁酯
(S)-1-BOC-3-(甲氨基)吡咯烷,96%
1-BOC-3-甲氨基吡咯烷(CAS号:454712-26-6)
N-BOC-3-Methylaminopyrrolidine
1-Boc-3-methylaminopyrrolidine ,98%
1-Boc-3-Methylaminopyrrolidine USP/EP/BP
1-(tert-Butyloxycarbonyl)-3-methylaminopyrro
3-Methylamino-1-tert-butoxycarbonylpyrrolidine
1-(tert-Butyloxycarbonyl)-3-methylaminopyrrolidine
tert-butyl 3-(methylamino)pyrrolidine-1-carboxylate
3-METHYLAMINO-PYRROLIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
3-(Methylamino)-1-pyrrolidinecarboxylic acid, 1,1-dimethylethyl ester
物理化学性质
制备方法
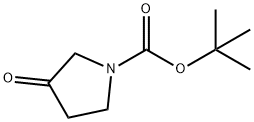
101385-93-7
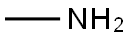
74-89-5

454712-26-6
一般步骤:在0℃下,向1-(叔丁氧基羰基)吡咯烷-3-酮(74 g,0.4 mol)的甲醇(450 mL)溶液中缓慢加入甲胺(137.8 g,1.2 mol)的甲醇溶液。将反应混合物在室温下搅拌2小时。随后,分批加入硼氢化钠(15.2 g,0.4 mol),并在室温下继续搅拌1小时。反应完成后,将混合物减压浓缩以除去溶剂,得到粗产物。将粗产物在二氯甲烷(3×500 mL)和水(300 mL)之间进行分配萃取。合并有机层,用无水硫酸钠干燥,过滤后减压浓缩,得到1-(叔丁氧基羰基)-3-甲氨基吡咯烷(74 g,收率92.5%),该产物无需进一步纯化即可用于下一步反应。产物结构经1H NMR(400 MHz,CDCl3)确认:δ 3.54(m,4H),3.29(m,2H),2.45(s,3H),2.20(m,1H),1.38(s,9H)。
参考文献:
[1] Patent: WO2009/76387, 2009, A1. Location in patent: Page/Page column 42
[2] Patent: US2015/252021, 2015, A1. Location in patent: Paragraph 0307; 0308
[3] Patent: WO2012/127506, 2012, A1. Location in patent: Page/Page column 108-109
[4] ChemCatChem, 2018, vol. 10, # 3, p. 510 - 514
[5] Patent: WO2007/122410, 2007, A1. Location in patent: Page/Page column 101; 106
