479630-08-5
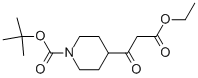 479630-08-5 结构式
479630-08-5 结构式
基本信息
N-BOC-4-(2-乙氧羰基乙酰基)哌啶
3-氧代-3-(1-BOC-4-哌啶基)丙酸乙酯
叔丁基4-(3-乙氧基-3-氧代丙酰)哌啶-1-甲酸酯
4-(3-乙氧基-3-氧代丙酰基)哌啶-1-甲酸叔丁酯
1-[(1,1-二甲基乙氧基)羰基]-BETA-氧代-4-哌啶丙酸乙酯
FB-0733(CAS:479630-08-5)N-BOC-4-(2-乙氧羰基乙酰基)哌啶
4-(2-ETHOXYCARBONYL-ACETYL)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER N-BOC-4-(2-乙氧羰基乙酰基)哌啶
1-Boc-4-(2-Ethoxycarbonyl-acetyl)
4-(3-Ethoxy-3-oxopropanoyl)piperidine
BOC-4-(3-ETHOXY-3-OXOPROPAN OYL)PIPERIDINE
1-Boc-4-(2-Ethoxycarbonyl-acetyl)piperidine
Ethyl 3-Oxo-3-(1-Boc-4-piperidyl)propanoate
N-BOC-4-(2-ETHOXYCARBONYL-ACETYL)-PIPERIDINE
1-Boc-b-oxo-4-piperidinepropanoic acid ethyl ester
4-(3-Ethoxy-3-oxopropanoyl)piperidine, N-BOC protected
N-(T-BUTOXYCARBONYL)-4-(2-ETHOXYCARBONYL-ACETYL) PIPERIDINE
物理化学性质
制备方法

64-17-5

1336874-02-2

479630-08-5
以乙醇和化合物(CAS:1336874-02-2)为原料合成4-(3-乙氧基-3-氧代丙酰基)哌啶-1-甲酸叔丁酯的一般步骤:将4-(3-乙氧基-3-氧代丙酰基)哌啶-1-甲酸叔丁酯(3)加入无水乙醇(200mL)中,并将溶液回流48小时。反应完成后,将溶液在真空下浓缩,并通过快速色谱法(洗脱剂:二氯甲烷)纯化,得到产物3,为微红色油状物(14.36g,收率85%)。产物表征数据如下:1H NMR(500MHz,CDCl3)δ 12.09(s,0.14H,烯醇OH),4.89(s,0.14H,烯醇CH),4.13(q,J = 7Hz,2H),4.10-3.96(m,2H),3.42(s,2H),2.81-2.67(m,2H),2.62-2.52(m,1H),1.85-1.71(m,2H),1.55-1.43(m,2H),1.39(s,9H),1.21(t,J = 7Hz,3H);13C NMR(125MHz,CDCl3)δ 204.0, 180.2(烯醇),172.7(烯醇),167.0, 154.4, 87.52, 79.51, 61.3, 48.5, 47.1, 28.2, 27.1, 13.9;薄层色谱Rf = 0.2(二氯甲烷)。高分辨质谱(HRMS)计算值C15H26NO5(M + H)+ 300.1805,实测值300.1808。
参考文献:
[1] Journal of Medicinal Chemistry, 2011, vol. 54, # 20, p. 7193 - 7205
[2] Patent: WO2013/25939, 2013, A2. Location in patent: Page/Page column 69; 71
