51419-59-1
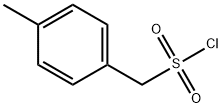 51419-59-1 结构式
51419-59-1 结构式
基本信息
(4-甲基苯基)甲烷磺酰氯
4-甲基苄磺酰氯
(4-甲基苯)甲烷磺酰氯
(4-甲苯基)甲烷黄酰氯,TECH,97%
4-METHYLBENZYLSULFONYL CHLORIDE
(4-METHYLPHENYL)METHANESULFONYL CHLORIDE
[(4-METHYLPHENYL)METHYL]SULFONYL CHLORIDE
P-METHYL-ALPHA-TOLUENESULFONYL CHLORIDE
P-TOLYL-METHANESULFONYL CHLORIDE
[(4-methylphenyl)methyl]sulphonyl chloride
4-Methylbenzylsulphonyl chloride, alpha-p-Xylenesulphonyl chloride
ɑ-p-Xylenesulfonyl chloride, 97%
p-Methyl-α-toluenesulfonyl chloride
(4-Methylphenyl)methanesulfonyl chloride, 4-Methylbenzenemethanesulfonyl chloride, p-Methyl-α-toluenesulfonyl chloride, p-Tolylmethanesulfonyl chloride
^a-p-Xylenesulfonyl chloride, 97%
物理化学性质
安全数据
制备方法
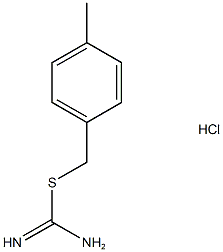
940-63-6

51419-59-1
通用方法:将烷基卤(或硫酸盐)(5mmol)与硫脲(0.381g,5mmol)在乙醇(5mL)中回流加热1小时。反应完成后,在真空条件下除去溶剂,并用乙醚(3×5mL)洗涤残余物,得到相应的S-烷基异硫脲盐,为白色固体,收率接近定量。无需进一步纯化,将所得产物转移至配备温度计和加料漏斗的三颈圆底烧瓶中,置于冰浴中。随后加入水(0.45mL)和乙腈(10mL)。在剧烈搅拌下,向混合物中缓慢滴加叔丁基次氯酸盐(2.86mL)的乙腈(5mL)溶液,控制反应温度在0-20℃范围内。滴加完毕后,继续搅拌反应混合物30分钟。反应结束后,在真空条件下除去溶剂,加入乙醚(15mL)溶解残余物,用水(2×10mL)洗涤有机相,无水硫酸钠干燥,最后在真空下浓缩,得到高纯度的4-甲基苄磺酰氯。产物可通过石油醚-乙酸乙酯混合溶剂重结晶进一步纯化。
参考文献:
[1] Synthesis (Germany), 2015, vol. 47, # 20, p. 3186 - 3190
[2] European Journal of Organic Chemistry, 2012, # 26, p. 5028 - 5037,10
[3] European Journal of Organic Chemistry, 2012, # 26, p. 5028 - 5037
[4] Journal of the American Chemical Society, 1987, vol. 109, # 24, p. 7472 - 7477
[5] Synthesis (Germany), 2013, vol. 45, # 12, p. 1675 - 1682

