54127-30-9
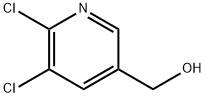 54127-30-9 结构式
54127-30-9 结构式
基本信息
5,6-二氯-3-吡啶甲醇
5,6-二氯吡啶-3-甲醇
6-二氯吡啶-3-基)甲醇
3-二氯-5-羟基甲基嘧啶
(5,6-二氯吡啶-3-基)甲醇
5,6-DICHLORONICOTINYL ALCOHOL
5,6-DICHLORO-3-PYRIDINEMETHANOL
2,3-Dichloro-5-pyridinemethanol
(5,6-Dichloro-3-pyridinyl)methanol
(5,6-dichloropyridin-3-yl)methanol
5,6-dichloro-3-pyridinemethanol96+%
2,3-DICHLORO-5-HYDROXYMETHYLPYRIDINE
2,3-Dichloro-5-(hydroxymethyl)pyridine 95+%
2,3-Dichloro-5-hydroxymethylpyridine 5,6-Dichloronicotinyl Alcohol
物理化学性质
制备方法
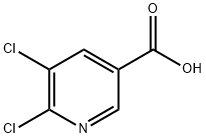
41667-95-2

54127-30-9
以5,6-二氯吡啶-3-羧酸为原料合成5,6-二氯-3-嘧啶甲醇的一般步骤如下:在0℃条件下,将859 mL(859 mmol)1M硼烷/四氢呋喃络合物的四氢呋喃溶液缓慢滴加到110 g(573 mmol)5,6-二氯烟酸的250 mL四氢呋喃溶液中。反应混合物逐渐升温至室温,并在该温度下持续搅拌3小时。反应完成后,将混合物冷却至0℃,随后用饱和碳酸钾水溶液调节至碱性。在旋转蒸发器上移除大部分四氢呋喃,残余物用乙酸乙酯多次萃取。合并有机相,依次用水和饱和氯化钠水溶液洗涤,然后用硫酸钠干燥。有机相在减压下浓缩,所得残余物通过硅胶柱色谱(硅胶60-Merck,粒径:0.04至0.063 mm)纯化,流动相为乙酸乙酯:环己烷(1:2),最终得到62 g(产率61%)的(5,6-二氯吡啶-3-基)甲醇。产物经1H-NMR(CD3CN)表征:δ[ppm] = 3.31(t, 1H), 4.60(d, 2H), 7.85(s, 1H), 8.26(s, 1H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2014, vol. 57, # 13, p. 5620 - 5637
[2] Patent: US2009/181947, 2009, A1. Location in patent: Page/Page column 39
[3] Patent: US2009/247551, 2009, A1. Location in patent: Page/Page column 27
[4] Patent: US2010/48646, 2010, A1. Location in patent: Page/Page column 25
[5] Patent: US6737435, 2004, B1. Location in patent: Page/Page column 18
