57477-39-1
 57477-39-1 结构式
57477-39-1 结构式
基本信息
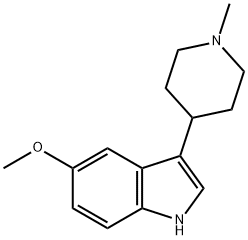
3-(1-甲基-4-哌啶基)-1H-吲哚-5-醇
BRL 54443
BRL 54443 HCl
BRL 54443, >=98%
BRL-54443
BRL54443
BRL 54443 USP/EP/BP
BRL54443
BRL 54443
BRL-54443
3-(1-methylpiperidin-4-yl)-1H-indol-5-ol
3-(1-Methyl-4-piperidinyl)-1H-indol-5-ol
1H-Indol-5-ol, 3-(1-Methyl-4-piperidinyl)-
物理化学性质
制备方法
111963-87-2

57477-39-1
1. 将5-甲氧基-3-(1-甲基-4-哌啶基)吲哚(2.30 g,9.4 mmol)溶解于30 mL 30%氢溴酸乙酸溶液中。 2. 将反应混合物置于密封管中,于105℃加热72小时。 3. 反应完成后,冷却至室温,并进行真空浓缩以去除溶剂。 4. 将浓缩后的残余物溶解于水中,用5N氢氧化钠水溶液调节pH至约13。 5. 再次进行真空浓缩,将残余物通过硅胶柱色谱纯化,使用20% 2M氨的甲醇/二氯甲烷溶液作为洗脱剂。 6. 收集目标组分,真空浓缩后,将残余物溶解于甲醇中。 7. 加入Dowex? 50X8-200离子交换树脂(25 g),室温下搅拌过夜。 8. 过滤混合物,依次用水和甲醇洗涤树脂。 9. 将树脂与100 mL 2M氨的甲醇溶液混合,搅拌过夜后过滤。 10. 将滤液真空浓缩,得到1.84 g(产率85%)的3-(1-甲基哌啶-4-基)-1H-吲哚-5-醇,无需进一步纯化即可用于后续步骤。 11. 元素分析结果:对于C13H17N3O,计算值:C,67.53;H,7.36;N,18.18。实测值:C,67.24;H,7.37;N,18.38。
参考文献:
[1] Patent: US6358972, 2002, B1
常见问题列表
|
5-HT 1E Receptor 1.1 nM (Ki) |
5-HT 1F Receptor 0.7 nM (Ki) |
5-HT 1A Receptor 63 nM (Ki) |
5-HT 1B Receptor 126 nM (Ki) |
5-HT 1D Receptor 63 nM (Ki) |
5-HT 2A Receptor 1259 nM (Ki) |
5-HT 2B Receptor 100 nM (Ki) |
5-HT 2C Receptor 316 nM (Ki) |
5-HT 6 Receptor >10,000 nM (Ki) |
5-HT 7 Receptor >10,000 nM (Ki) |
Despite its low affinity for other receptors [5-HT
1A
(63 nM), 5-HT
1B
(126 nM), 5-HT
1D
(63 nM), 5-HT
2A
(1259 nM), 5-HT
2B
(100 nM), 5-HT
2C
(316 nM), 5-HT
6
(>10,000 nM), 5-HT
7
(>10,000 nM), D
2
(501 nM), D
3
(631 nM), and α
1B
-adrenoceptors (1259 nM)], BRL54443 binds with high affinity at 5-HT
1F
receptors.
In DG membranes, BRL54443 selectively stimulates 5-HT
1E
receptors and potently inhibits forskolin-dependent cAMP production (IC
50
=14 nM). BRL 54443 also induces contraction (-log EC
50
=6.52).
Reduction of flinching was considered as antinociception. Ipsilateral, but not contralateral, peripheral administration of BRL54443 (5-HT(1E/1F); 3-300 microg/paw) significantly reduced formalin-induced flinching in rats.