6081-61-4
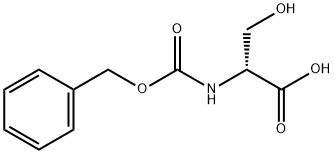 6081-61-4 结构式
6081-61-4 结构式
基本信息
N-苄氧羰基-D-丝氨酸, 98+%
CBZ-D-SER-OH
N-ALPHA-CARBOBENZOXY-D-SERINE
N-ALPHA-CBZ-D-SERINE
N-BENZYLOXYCARBONYL-D-SERINE
N-CARBOBENZOXY-D-SERINE
N-CBZ-D-SERINE
Z-D-SERINE
Z-D-SER-OH
N-Benzyloxycarbonyl-D-serine,98+%
CBZ-D-SERINE
N-(Carbobenzyloxy)-D-serine
Z-D-SERINE extrapure
(2R)-2-(Benzyloxycarbonylamino)-3-hydroxypropionic acid
(R)-2-[[(Benzyloxy)carbonyl]amino]-3-hydroxypropinic acid
(R)-3-Hydroxy-2-(benzyloxycarbonylamino)propionic acid
物理化学性质
制备方法
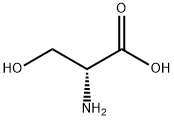
312-84-5

501-53-1

6081-61-4
一般步骤:将碳酸氢钠(605 g,5.72 mol)溶于水(3 L)中,冰浴冷却至0-5℃。分批加入D-丝氨酸(200 g,1.90 mol),分8-10份,每次间隔20-30分钟。随后,在1小时内缓慢滴加50%氯甲酸苄酯的甲苯溶液(780 g,2.29 mol),保持温度在0-5℃。反应混合物在0-5℃下搅拌2小时,然后升至室温继续搅拌2小时。加入乙酸乙酯(400 mL),用盐酸调节pH至2.0。用乙酸乙酯(2×800 mL)萃取产物。合并有机相,减压浓缩至约640 mL,得到悬浮液。加入环己烷(1200 mL),浆化6小时。过滤收集纯化产物,50℃减压干燥,得到(R)-2-(((苄氧基)羰基)氨基)-3-羟基丙酸(432.5 g,95%收率),为白色固体,熔点119-120℃(文献值119℃),[α]D25 -5.08(c 2.7,乙酸)。IR(ν, cm-1):1689(C=O),1749(C=O),3319(NH),3338(OH)。UV(λmax, nm):208。1H NMR(CD3OD, δ, ppm, J/Hz):3.83-3.92(2H, m, CH2),4.30(1H, t, J=8.8, NCH),5.13(2H, s, CH2),7.29-7.40(5H, m, Ph-H)。13C NMR(CD3OD, δ, ppm):57.7,63.1,67.7,128.9(2C),129.0,129.5(2C),138.1,158.6,173.8。质谱(m/z, Irel%):238 [M-H]-(100)。
参考文献:
[1] Tetrahedron Letters, 2014, vol. 55, # 19, p. 3114 - 3116
[2] Chemistry of Heterocyclic Compounds, 2017, vol. 53, # 11, p. 1248 - 1253
[3] Khim. Geterotsikl. Soedin., 2017, vol. 53, # 11, p. 1248 - 1253,6
[4] European Journal of Organic Chemistry, 2001, # 16, p. 3067 - 3074
[5] Asian Journal of Chemistry, 2011, vol. 23, # 11, p. 5169 - 5170
