6093-71-6
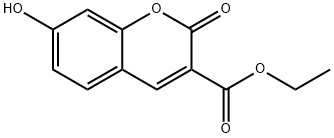 6093-71-6 结构式
6093-71-6 结构式
基本信息
7-羟基香豆素-3-羧酸乙酯
7-羟基香豆素-3-甲酸乙酯
7-羟基-2-氧代-2H-苯并吡喃-3-羧酸乙酯
AURORA 9226
AKOS BBS-00008132
3-CARBETHOXYUMBELIFERONE
ETHYL 3-UMBELLIFERYLCARBOXYLATE
ethyl umbelliferone-3-carboxylate
Ethyl 7-Hydroxycoumarin-3-carboxylate
Ethyl7-Hydroxycoumarin-3-carboxylate>
ethyl 7-hydroxy-2-oxochromene-3-carboxylate
UMBELLIFERONE 3-CARBOXYLIC ACID ETHYL ESTER
物理化学性质
制备方法

105-53-3
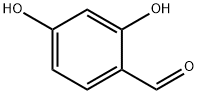
95-01-2

6093-71-6
以2,4-二羟基苯甲醛(3.70 mmol)和丙二酸二乙酯(7.90 mmol)为原料,合成7-羟基-2-氧代-2H-苯并吡喃-3-羧酸乙酯的一般步骤如下:向2,4-二羟基苯甲醛的丙二酸二乙酯溶液中加入哌啶(10滴),所得混合物于室温下搅拌反应2小时。反应完成后,用10%盐酸水溶液(5 mL)酸化反应混合物。析出的固体经过滤收集,并用冷水(10 mL)洗涤。粗产物通过快速柱色谱法纯化,以二氯甲烷:乙酸乙酯(70:30,v/v)为洗脱剂,得到目标化合物7-羟基-2-氧代-2H-苯并吡喃-3-羧酸乙酯(0.735 g,收率97%)。产物结构经1H NMR(400 MHz, DMSO-d6)和13C NMR(100 MHz, DMSO-d6)确认:1H NMR δ 1.28 (t, J = 7.1 Hz, 3H), 4.24 (q, J = 7.1 Hz, 2H), 6.71 (d, J = 1.8 Hz, 1H), 6.83 (dd, J = 1.8, 8.3 Hz, 1H), 7.74 (d, J = 8.3 Hz, 1H), 8.66 (s, 1H), 11.07 (s, 1H); 13C NMR δ 14.1, 60.8, 101.7, 110.4, 113.9, 120.0, 132.1, 149.4, 156.3, 157.0, 162.9, 164.0。
参考文献:
[1] Tetrahedron Letters, 2012, vol. 53, # 22, p. 2715 - 2718
[2] Patent: KR101744655, 2017, B1. Location in patent: Paragraph 0058-0060
[3] Patent: CN106279125, 2017, A. Location in patent: Paragraph 0039; 0040
[4] Letters in Organic Chemistry, 2013, vol. 10, # 7, p. 468 - 477
[5] Chemical Communications, 2017, vol. 53, # 11, p. 1813 - 1816
