61-70-1
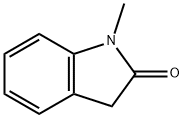 61-70-1 结构式
61-70-1 结构式
基本信息
N-甲基吗啉氧化物
硝苯地平酸
1-METHYL-2-INDOLINONE
1-METHYLOXINDOLE
2,3-DIHYDRO-1-METHYLINDOL-2-ONE
N-METHYL-2-OXINDOLE
N-METHYLOXINDOLE
NMO
TIMTEC-BB SBB006878
1,3-dihydro-1-methyl-2h-indol-2-on
1,3-dihydro-1-methyl-2h-indol-2-one
1-Methyl-1,3-dihydro-indol-2-one
1-methyl-2-indolinon
1-methyl-oxindol
ba2777
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl) -3,5-pyridinedicarboxylic acid,monomethylester
1,4-DIHYDRO-2,6-DIMETHYL-4-(3-NTIROPHENYL)-3,5-PYRIDINEDICARBOXYLIC ACID MONOMET
1,4-DIHYDRO-5-METHOXYCARBONYL-2,6-DIMETHYL-4-(3-NITROPHENYL)PYRIDINE-3-CARBOXYLIC ACID
methyl hydrogen 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate
5-METHOXYCARBONYL-2,6-DIMETHYL-4(3-NITROPHENYL)-1,4-DIHYDRPYRIDINE-3-CARBOXYLIC ACID(LERCANIDIPINE INTERMEDIATE)
rac-1,4-Dihydro-5-(methoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-3-pyridinecarboxylic Aci
物理化学性质
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
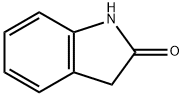
59-48-3

77-78-1

61-70-1
向5 L四颈烧瓶(配备机械搅拌器、冷凝管和氮气入口)中加入2 L水和50%氢氧化钠溶液(2.52 mol, 201.6 g, 2.25当量),随后加入2-吲哚酮(1.12 mol, 150 g, 1当量)。将反应混合物加热至40℃。通过注射器缓慢滴加硫酸二甲酯(1.68 mol, 211.7 g, 159 mL, 1.5当量),滴加过程中反应放热,温度升至53℃。滴加完毕后,将反应混合物加热至100℃并维持15分钟。待反应混合物冷却至60℃后,加入第二部分硫酸二甲酯(0.476 mol, 60 g, 45 mL, 0.425当量),再次将反应混合物加热至100℃并保持15分钟。通过TLC(展开剂:庚烷/乙酸乙酯,1:1)监测反应进度,确认甲基化反应基本完成。将反应混合物冷却至50℃,用浓盐酸调节pH至约7。接种晶种后,将混合物冷却至室温并静置过夜。过滤收集固体产物,用水洗涤四次(4×),随后在40℃下真空干燥过夜,得到1-甲基-2-吲哚啉酮110.7 g(收率67%),为粉红色固体,熔点为84-86℃。
参考文献:
[1] Organic Letters, 2012, vol. 14, # 10, p. 2544 - 2547
[2] Tetrahedron Letters, 2015, vol. 56, # 26, p. 3992 - 3995
[3] Chemistry - An Asian Journal, 2017, vol. 12, # 7, p. 734 - 743
[4] Journal of Organic Chemistry, 2010, vol. 75, # 4, p. 1047 - 1060
[5] Tetrahedron Letters, 2001, vol. 42, # 41, p. 7315 - 7317
