65271-80-9
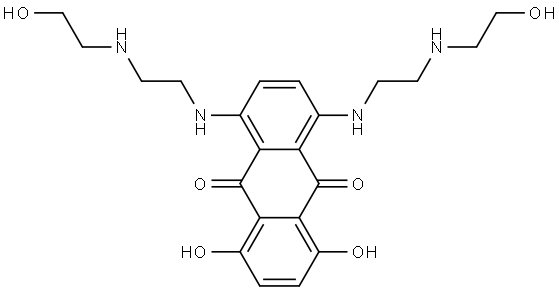 65271-80-9 结构式
65271-80-9 结构式
基本信息
米托蒽醌
米托恩醌
MITOXANTRONE
1,4-dihydroxy-5,8-bis(2-((2-hydroxyethyl)amino)ethylamino)-9,10-anthracenedi
5,8-bis((2-((2-hydroxyethyl)amino)ethyl)amino)-1,4-dihydroxy-anthraquinon
5,8-bis((2-((2-hydroxyethyl)amino)ethyl)amino)-1,4-dihydroxyanthraquinone
9,10-anthracenedione,1,4-dihydroxy-5,8-bis((2-((2-hydroxyethyl)amino)ethyl)a
dihydroxyanthraquinone
mitoxanthrone
nsc279836
MitoxantroneBase
Mitoxantrone Hcl 70476-82-3 / Base
PharmasubstanceEP4
1,4-Dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-9,10-anthracenedione
NSC-27983
9,10-Anthracenedione, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-
1,4-dihydroxy-5,8-bis(2-((2-hydroxyethyl)amino)ethylamino)-9,10-anthrace nedimitoxantrone
MITOXANTRONUM AND THE INTERMEDIATES
Mitoxantrone (base and/or unspecified salts)
MITOXANTRONUM
1,4-Dihydroxy-5,8-bis[2-[(2-hydroxyethyl)amino]ethylamino]-9,10-anthraquinone
物理化学性质
制备方法
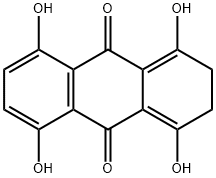
81-59-4
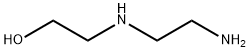
111-41-1

65271-80-9
1. 在室温下,于250 mL三颈烧瓶中加入10.5 g 1,4,5,8-四羟基-2,3-二氢蒽-9,10-二酮,随后在氮气或氩气保护下对烧瓶进行抽真空处理。 2. 抽真空后,向烧瓶中通入空气,加入59 mL 1,4-二恶烷并启动搅拌,使1,4,5,8-四羟基-2,3-二氢蒽-9,10-二酮完全溶解。 3. 将2.38 g N-(2-羟乙基)乙二胺加入恒压滴液漏斗中,缓慢滴加到上述溶液中,滴加过程中持续搅拌,滴加时间为14分钟,滴加完毕后得到糊状粘稠液体。 4. 将反应混合物置于53℃水浴中加热,反应2.2小时,反应结束后得到蓝棕色或蓝紫色油状液体,记为产物D。 5. 将产物D转移至带塞的容器中,加入170 mL无水乙醇,记录液体体积。将容器置于59℃水浴中,通入经无水硫酸钙干燥的氧气,缓慢氧化5.5小时,直至反应液变为亮蓝色。 6. 将亮蓝色液体冷却至室温,补充乙醇至记录的体积,室温静置30小时后,进行真空过滤,得到蓝色晶体产物E。 API的精制步骤: 1. 在烧杯中按绝对乙醇与正己烷体积比3:1配制180 mL混合溶剂。 2. 称取9 g产物E,加入三颈瓶中,倒入上述混合溶剂,54℃水浴中搅拌至完全溶解。加入活性炭,持续搅拌并加热至68℃回流22分钟。热回流结束后立即减压过滤,滤液冷却后冰浴过夜结晶,真空过滤得到药物晶体。 3. 重新配制混合溶剂,用其对药物晶体进行多次洗涤,直至获得无可见红棕色、具有清晰光泽且粒度均匀的蓝色晶体。洗涤完成后,将晶体于105℃恒温干燥箱中干燥1.9小时,得到精制药物盐酸米托蒽醌。通过HPLC-UV测定,收率为38%。
参考文献:
[1] Patent: CN108395379, 2018, A. Location in patent: Paragraph 0023; 0025-0077
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-13502 | 米托蒽醌 Mitoxantrone | 65271-80-9 | 25 mg | 371元 |
| 2025/12/22 | HY-13502 | 米托蒽醌 Mitoxantrone | 65271-80-9 | 10mM * 1mLin DMSO | 408元 |
| 2025/12/22 | HY-13502 | 米托蒽醌 Mitoxantrone | 65271-80-9 | 50mg | 515元 |
常见问题列表
|
PKC 8.5 μM (IC 50 ) |
Topoisomerase II
|
Mitoxantrone inhibits PKC in a competitive manner with respect to histone H1, and its K i value is 6.3 μM and in a non-competitive manner with respect to phosphatidylserine and ATP. Treatment of B-CLL cells for 48 h with mitoxantrone (0.5 μg/mL) induces a decrease in cell viability. Mitoxantrone induces DNA fragmentation and the proteolytic cleavage of poly(ADP-ribose) polymerase (PARP), demonstrating that the cytotoxic effect of mitoxantrone is due to induction of apoptosis. Mitoxantrone shows cytotoxicity to human breast carcinoma cell lines MDA-MB-231 and MCF-7 with IC 50 values of 18 and 196 nM, respectively.
Mitoxantrone given IP at the optimal dose (1.6 mg/kg/day; as a free base) produces a statistically significant number of 60-day survivors (curative effect) in mice with IP implanted L1210 leukemia. In SC implanted Lewis lung carcinoma, mitoxantrone and ADM administered IV also shows effective antitumor activities and produces a 60% and a 45% ILS, respectively..

