6972-27-6
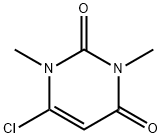 6972-27-6 结构式
6972-27-6 结构式
基本信息
1,3-二甲基-6-氯尿嘧啶
6-氯-1,3-二甲基尿嘧啶
6-CHLORO-1,3-DIMETHYL-2,4(1H,3H)-PYRIMIDINEDIONE
6-CHLORO-1,3-DIMETHYLPYRIMIDINE-2,4(1H,3H)-DIONE
6-CHLORO-1,3-DIMETHYLURACIL
AURORA KA-4432
1,3-Dimethyl-4-chlorouracil
2,4(1H,3H)-Pyrimidinedione, 6-chloro-1,3-dimethyl-
4-Chloro-1,3-dimethyluracil
6-Chloro-1,3-dimethyl
6-CHLORO-1,3-DIMETHYLURACIL 99%
6-CHLORO-1,3-DIMETHYLPYRIMIDINE-2,6-1H,3H-DIONE
6-Chloro-1,3-dimethylpyrimidine-2,4-dione
1,3-Dimethyl-6-chloro-1,2,3,4-tetrahydropyrimidine-2,4-dione
物理化学性质
制备方法
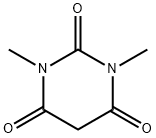
769-42-6

6972-27-6
以1,3-二甲基巴比妥酸(化合物SM1)(7 kg,44.9 mmol)为原料,将其溶于120 kg磷酰氯(POCl3)中。缓慢滴加水(2.2 kg,122 mol)至上述溶液中。滴加完毕后,缓慢加热至回流,维持反应6小时;反应进程通过高效液相色谱(HPLC)监测。反应完成后,将反应液缓慢倒入大量冰水(100 L)中,随后用二氯甲烷(50 L)萃取两次,合并有机相。有机相用饱和盐水(20 L)洗涤,无水硫酸钠干燥,过滤后浓缩有机相,最后通过真空干燥得到黄色固体产物6-氯-1,3-二甲基尿嘧啶(化合物SM2)(7.2 kg),收率为92.3%。
参考文献:
[1] Chemistry of Heterocyclic Compounds (New York, NY, United States), 1987, vol. 23, # 6, p. 690 - 697
[2] Khimiya Geterotsiklicheskikh Soedinenii, 1987, vol. 23, # 6, p. 836 - 844
[3] Patent: CN103755705, 2017, B. Location in patent: Paragraph 0033; 0034; 0035
[4] Justus Liebigs Annalen der Chemie, 1958, vol. 612, p. 158,162
[5] Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1995, vol. 34, # 10, p. 869 - 871
