723281-72-9
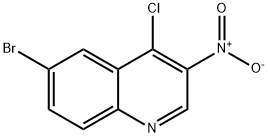 723281-72-9 结构式
723281-72-9 结构式
基本信息
3-硝基-4-氯-6-溴喹啉
6-溴-4-氯-3-硝基喹啉 100G
Quinoline, 6-broMo-4-chloro-3-nitro-
物理化学性质
制备方法
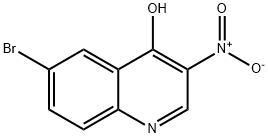
853908-50-6

723281-72-9
步骤3:将6-溴-4-羟基-3-硝基喹啉(步骤2的产物,20 g,74.3 mmol)与三氯氧磷(POCl3,150 mL,1613 mmol)混合,搅拌反应45分钟。反应完成后,将混合物冷却至室温,并缓慢倒入冰水中以淬灭反应。通过过滤收集析出的固体,用冰冷的水洗涤,随后将固体溶解于二氯甲烷(CH2Cl2)中。有机层用冷的饱和食盐水洗涤,并用无水硫酸钠(Na2SO4)干燥。减压蒸发除去溶剂,得到6-溴-4-氯-3-硝基喹啉。产量:8 g(38%);1H NMR(CDCl3,500 MHz):δ 9.275(s,1H),8.611-8.615(d,1H,J = 2 Hz),8.100-8.118(d,1H,J = 9 Hz),8.026-8.048(dd,1H,J = 8.5 Hz,2 Hz)。
参考文献:
[1] Patent: US2015/320727, 2015, A1. Location in patent: Paragraph 0555; 0556
[2] Patent: WO2015/145369, 2015, A1. Location in patent: Page/Page column 38
[3] Patent: WO2009/155527, 2009, A2. Location in patent: Page/Page column 142
[4] MedChemComm, 2016, vol. 7, # 2, p. 297 - 310
[5] Patent: CN108456165, 2018, A. Location in patent: Paragraph 0047; 0081-0082
