77156-85-5
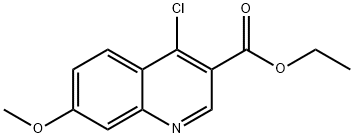 77156-85-5 结构式
77156-85-5 结构式
基本信息
4-氯-7-甲氧基喹啉-3-羧酸乙酯
4-氯-7-甲氧基-3-喹啉羧酸乙酯
4-氯-7-甲氧基-喹啉-3-甲酸乙酯
3-氯-7-甲氧基-喹啉-3-甲酸甲酯
乙基 4-氯-7-甲氧基喹啉-3-羧酸酯
乙基4-氯-7-甲氧基喹啉-3-羧酸酯,97%
Ethyl 4-chloro-7-methoxyquinoline- 3-carboxylate
Ethyl 4-chloro-7-Methoxyquinoline-3-caroboxylate
Ethyl4-chloro-7-methoxyquinoline-3-carboxylate,97%
4-Chloro-7-methoxy-quinoline-3-carboxylic acid ethyl ester
4-Chloro-7-methoxy-quinoline-3-carboxylic acid methyl ester
3-Quinolinecarboxylic acid, 4-chloro-7-methoxy-, ethyl ester
制备方法
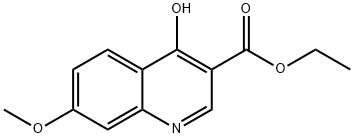
63463-15-0

77156-85-5
步骤C. 4-氯-7-甲氧基喹啉-3-羧酸乙酯的合成。 将4-羟基-7-甲氧基喹啉-3-羧酸乙酯(2.72g,11.0mmol)溶解于氯仿(100mL)中,随后加入草酰氯(2.93mL,33.0mmol)和5滴N,N-二甲基甲酰胺(DMF)。 反应混合物在65℃下加热回流2.5小时,之后冷却至室温。 将反应液倒入饱和碳酸氢钠水溶液中,用二氯甲烷(DCM)萃取有机相。 合并有机相,用无水硫酸镁干燥,过滤后减压浓缩,得到目标产物4-氯-7-甲氧基喹啉-3-羧酸乙酯,为黄色固体(2.69g,收率92%)。 产物经1H NMR(400MHz,CDCl3)表征:δ9.20(s,1H),8.33(d,J = 9.4Hz,1H),7.58(br.s,1H),7.37(d,J = 9.0Hz,1H),4.48(q,J = 6.8Hz,2H),4.01(s,3H),1.45(t,J = 7.0Hz,3H)。质谱分析显示[M + H]+ =266.2。
参考文献:
[1] Patent: US2014/275548, 2014, A1. Location in patent: Paragraph 0361
[2] Patent: WO2016/196961, 2016, A1. Location in patent: Paragraph 00149
[3] Journal of Medicinal Chemistry, 2018, vol. 61, # 6, p. 2422 - 2446
[4] Patent: WO2018/129274, 2018, A1. Location in patent: Paragraph 0046
[5] Farmaco, 1998, vol. 53, # 8-9, p. 579 - 585
