81246-82-4
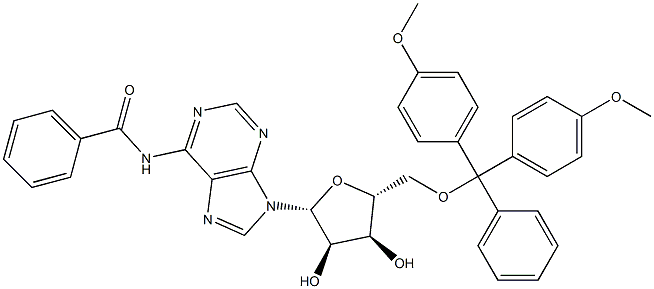 81246-82-4 结构式
81246-82-4 结构式
基本信息
N6-苯甲酰基-5'-DMT-腺苷
5'-O-(4,4'-二甲氧基三苯甲基)-N6-苯甲酰基腺苷
N6-苯甲酰基-5'-O-(4,4'-二甲氧基三苯甲基)-腺苷
5'-DMT-Bz-rA
5'-O-DMT-Bz-rA
5'-O-DMT-N6-Bz Adenosine
-DMT-RIBO ADENOSINE (N-BZ)
5'-DMT-RIBO ADENOSINE (N-BZ)
5'-O-DMTr-N6-benzoyladenosine
N6-Benzoyl-5'-O-DMT-adenosine
5'-DMT-RIBO ADENOSINE (N-BZ) USP/EP/BP
5'-O-dimethoxytrityl-N6-benzoyl adenosine
制备方法
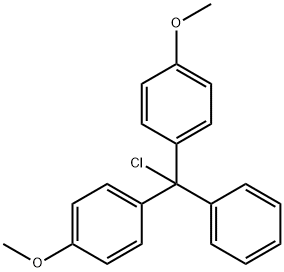
40615-36-9
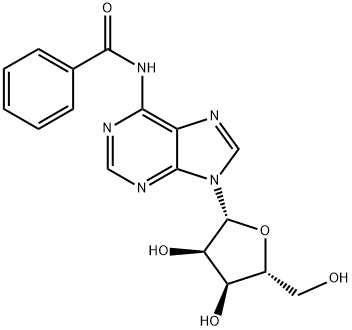
4546-55-8
![Benzamide, N-[9-[5-O-[bis(4-methoxyphenyl)phenylmethyl]-α-D-ribofuranosyl]-9H-purin-6-yl]-](/CAS/20210305/GIF/129666-75-7.gif)
129666-75-7
以4,4'-双甲氧基三苯甲基氯(DMTrCl)和N6-苯甲酰基腺苷为原料合成目标化合物(CAS:129666-75-7)的一般步骤如下:在0℃下,向含有N-(9-((2R,3R,4R,5R)-3,4-二羟基-5-(羟甲基)四氢呋喃-2-基)-9H-嘌呤-6-基)-苯甲酰胺(100g,269.3mmol)的吡啶溶液(500mL)中,依次加入DMAP(1.64g,13.46mmol,0.05当量)和DMTrCl(100.4g,296.2mmol,1.1当量)。将反应混合物在室温下搅拌16小时,反应完成后,通过加入甲醇(500mL)淬灭反应。减压蒸馏去除挥发性物质,残余物通过硅胶柱色谱法纯化(洗脱梯度:1/1石油醚/乙酸乙酯至100%乙酸乙酯),得到N-(9-((2R,3R,4S,5R)-5-((双(4-甲氧基苯基)(苯基)甲氧基)甲基)-3,4-二羟基四氢呋喃-2-基)-9H-嘌呤-6-基)苯甲酰胺,为白色泡沫状固体(150g,223mmol,收率83%)。
参考文献:
[1] Tetrahedron Asymmetry, 2005, vol. 16, # 17, p. 2908 - 2917
[2] Patent: WO2018/156625, 2018, A1. Location in patent: Paragraph 0295
[3] Canadian Journal of Chemistry, 1982, vol. 60, p. 1106 - 1113
[4] Patent: WO2017/161349, 2017, A1. Location in patent: Paragraph 0289
[5] Nucleosides, Nucleotides and Nucleic Acids, 2008, vol. 27, # 8, p. 1001 - 1008
