847818-70-6
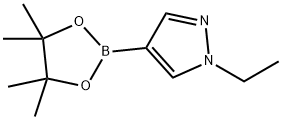 847818-70-6 结构式
847818-70-6 结构式
基本信息
1-乙基吡唑-4-硼酸频哪醇酯
1-乙基-4-吡唑硼酸频哪醇酯
1-乙基-1H-吡唑-4-硼酸频
1-乙基-1H-吡唑-4-硼酸频那
1-乙基-1H-吡唑-4-硼酸频哪醇酯
1-乙基-1H-吡唑-4-硼酸频那醇酯
1-乙基-4-(4,4,5,5-四甲基-1,3,2-二氧杂戊硼烷-2-基)-1H-吡唑
1-Ethyl-1H-pyrazole-4-boronic acid, pina
1-Ethylpyrazole-4-boronic Acid Pinacol Ester
1-Ethyl-1H-pyrazole-4-boronic acid, pinacol ester
1-ethyl-4-(tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1-Ethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole
1-ethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-p...
2-(1-Ethyl-1H-pyrazol-4yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
1-Ethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
1H-Pyrazole, 1-ethyl-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-
物理化学性质
制备方法
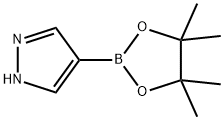
269410-08-4

75-03-6

847818-70-6
向4-(4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)-1H-吡唑(1.0g,5.2mmol)的N,N-二甲基甲酰胺(DMF,10mL)溶液中加入碳酸铯(2.5g,7.7mmol)和碘乙烷(1.2mL,15mmol)。将反应混合物在室温下搅拌12小时。反应完成后,用水(30mL)稀释反应混合物。用二氯甲烷(DCM,30mL×3)萃取水相。合并有机层,用无水硫酸钠(Na2SO4)干燥,随后在减压下浓缩。通过硅胶柱色谱法纯化残余物,使用石油醚(PE)/乙酸乙酯(EtOAc)(体积比2:1)作为洗脱剂,得到无色油状产物1-乙基-1H-吡唑-4-硼酸频哪醇酯(880mg,产率77%)。质谱(MS)分析(电喷雾电离,阳离子模式)显示m/z:223.25 [M + 1]+。
参考文献:
[1] Patent: WO2016/615, 2016, A1. Location in patent: Paragraph 00655
[2] Patent: EP1798229, 2007, A1. Location in patent: Page/Page column 130
[3] Patent: US2010/240634, 2010, A1. Location in patent: Page/Page column 67-68
[4] Patent: WO2018/102452, 2018, A2. Location in patent: Paragraph 425; 426
[5] Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 19, p. 5299 - 5302
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW0284781870606 | 1-乙基吡唑-4-硼酸频哪醇酯 | 847818-70-6 | 100G | 1957元 |
| 2025/12/22 | XW0284781870605 | 1-乙基吡唑-4-硼酸频哪醇酯 | 847818-70-6 | 25G | 548元 |
| 2025/12/22 | E1348 | 1-乙基-4-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)-1H-吡唑 1-Ethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole | 847818-70-6 | 1g | 75元 |
