88223-35-2
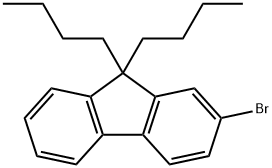 88223-35-2 结构式
88223-35-2 结构式
基本信息
2-Bromo-9,9-di-n-butylfluoren
2-Bromo-9,9-di-n-butylfluorene
2-BROMO-9,9-DI-N-BUTYLLFLUORENE
2-Bromo-9,9-dibutyl-9H-fluorene
9H-Fluorene, 2-bromo-9,9-dibutyl-
2-Bromo-9,9-di-n-butylfluoren ISO 9001:2015 REACH
物理化学性质
制备方法
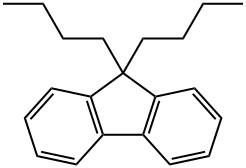
15069-42-8

88223-35-2
以9,9-二(正丁基)芴(26g,92mmol)为原料,与N-溴代琥珀酰亚胺(NBS,16.5g,92mmol)在丙酮(200mL)中混合。在氮气保护下,将反应混合物于80℃搅拌3小时。反应完成后,冷却至室温,将反应液倒入冷水中,并用二氯甲烷(DCM,3×200mL)萃取。合并有机层,用水洗涤,无水硫酸钠干燥,过滤后浓缩至干。粗产物通过硅胶短柱色谱法纯化,以石油醚为洗脱剂,得到目标产物2-溴-9,9-二丁基芴,为无色固体(29.2g,收率92.7%);熔点58-61℃。1H NMR(400MHz,CDCl3)δ(ppm):7.64-7.66(m,1H),7.50-7.55(m,1H),7.43-7.47(m,2H),7.31-7.34(m,3H),1.88-1.96(m,4H),1.03-1.12(m,4H),0.68(t,6H,J=7.4Hz),0.52-0.63(m,4H)。
参考文献:
[1] Australian Journal of Chemistry, 2008, vol. 61, # 7, p. 541 - 546
[2] Chemistry - A European Journal, 2005, vol. 11, # 11, p. 3285 - 3293
[3] Dyes and Pigments, 2012, vol. 95, # 3, p. 679 - 688
[4] New Journal of Chemistry, 2015, vol. 39, # 2, p. 1038 - 1044
[5] Patent: KR101567837, 2015, B1. Location in patent: Paragraph 0201; 0204; 0205; 0217
