918538-04-2
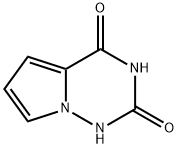 918538-04-2 结构式
918538-04-2 结构式
基本信息
吡咯并[2,1-F][1,2,4]三嗪-2,4(1H,3H)-2-酮
PYRROLO[2,1-F][1,2,4]TRIAZINE-2,4-DIONE
Pyrrolo[2,1-f][1,2,4]triazine-2,4(1H,3H)
1H-pyrrolo[2,1-f][1,2,4]triazine-2,4-dione
Pyrrolo[2,1-f][1,2,4]triazine-2,4(1H,3H)-dione
pyrrolo[1,2-f][1,2,4]triazine-2,4(1H,3H)-dione
PYRROLO[1,2-D][1,2,4]TRIAZINE-2,4(1H,3H)-DIONE
Pyrrolo[2,1-f][1,2,4]triazine-2,4(1H,3H)-dione (96%)
物理化学性质
制备方法
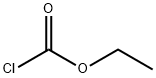
541-41-3
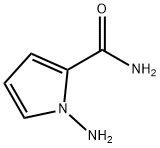
159326-69-9
![吡咯并[2,1-F][1,2,4]三嗪-2,4(1H,3H)-2-酮](/CAS/GIF/918538-04-2.gif)
918538-04-2
步骤13)吡咯并[2,1-f][1,2,4]三嗪-2,4(1H,3H)-二酮的合成:[0342] 在无水1,4-二恶烷(120mL)中悬浮1-氨基-1H-吡咯-2-甲酰胺(14.00g,0.11mol)和吡啶(9.90mL,0.12mol),随后加入氯甲酸乙酯(11.70mL,0.12mol)。将反应混合物在回流条件下搅拌1小时,然后进行真空浓缩。将残余物在155℃下继续搅拌17小时,冷却至室温后,用甲醇(50mL)稀释。将所得混合物在-15℃下搅拌2小时,过滤,收集固体产物,并在60℃下通过真空干燥器干燥过夜,得到灰色固体吡咯并[2,1-f][1,2,4]三嗪-2,4(1H,3H)-二酮(12.70g,收率75%)。质谱(ESI,阳离子模式)m/z:152.2 [M + H]+;1H NMR(400MHz,DMSO-d6):δ(ppm)12.96(s,1H),11.7(s,1H),7.13(m,1H),6.75(dd,J = 4.4,1.6Hz,1H),6.34(dd,J = 4.3,2.6 Hz,1H)。
参考文献:
[1] Patent: WO2016/190847, 2016, A1. Location in patent: Paragraph 0342
[2] Patent: CN104974163, 2017, B. Location in patent: Paragraph 0518; 0519; 0520; 0521
[3] Patent: WO2017/219800, 2017, A1. Location in patent: Paragraph 0082; 0083
[4] Patent: US2007/4731, 2007, A1. Location in patent: Page/Page column 15