Molnupiravir Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
EIDD-2801 is an orally bioavailable prodrug of the antiviral nucleoside derivative N4- hydroxycytidine (NHC, EIDD-1931). It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase. It exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. The broadspectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2. EIDD-2801 has the potential for COVID-19, seasonal and pandemic influenza treatment.
History
Molnupiravir was originally developed as antiviral therapy for influenza. However, this antiviral has also shown activity against various other viruses, including SARS-CoV-2. The drug co-developed by Emory University, Ridgeback Biotherapeutics, and Merck is a promising oral treatment for COVID-19. The international nonproprietary name of the drug was inspired by that of Thor's hammer, Mjölnir. The idea is that the drug will strike down the virus like a mighty blow from the god of thunder.
In 2019, the National Institute of Allergy and Infectious Diseases (NIAID) approved moving molnupiravir into Phase I clinical trials for influenza.
In 2020, a study found that it is orally active against SARS-CoV-2 in ferrets. DRIVE then licensed molnupiravir for human clinical studies to Miami-based company Ridgeback Biotherapeutics, which later partnered with Merck & Co. to develop the drug further.
Definition
Molnupiravir is a nucleoside analogue that is N(4)-hydroxycytidine in which the 5'-hydroxy group is replaced by a (2-methylpropanoyl)oxy group. It is the prodrug of the active antiviral ribonucleoside analog N(4)-hydroxycytidine (EIDD-1931), has activity against a number of RNA viruses including SARS-CoV-2, MERS-CoV, and seasonal and pandemic influenza viruses. It is currently in phase III trials for the treatment of patients with COVID-19. It has a role as a prodrug, an anticoronaviral agent and an antiviral drug. It is a nucleoside analogue, an isopropyl ester and a ketoxime. It derives from a N(4)-hydroxycytidine.
www.ebi.ac.uk
Biologische Aktivität
EIDD-2801 is an orally active, 5′-isopropylester prodrug form of the antiviral ribonucleoside analog N4-hydroxycytidine (NHC, EIDD-1931) with known anti-viral activity against SARS-CoV, SARS-CoV-2, RSV, influenza A & B (IAV & IBV), HCV, pestivirus, bovine viral diarrhoea virus (BVDV), Ebola (EBOV), Chikungunya (CHIKV), venezuelan equine encephalitis (VEEV). EIDD-2801 is efficiently hydrolyzed to NHC in vivo, being more orally bioavailable than NHC in nonhuman primates and ferrets, while maintaining similar oral bioavailability as NHC in mice. EIDD-2801 displays in vivo efficacy against pandemic and seasonal IAV strains in ferrets (lowest ED = 2.3 and 7 mg/kg b.i.d. p.o., respectively).
Synthese
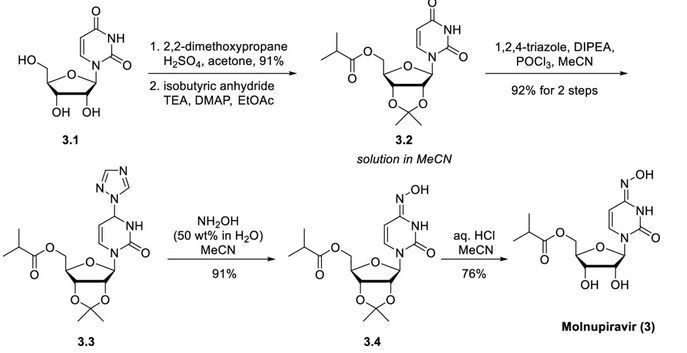
The synthesis of molnupiravir starts from uridine and is synthesized in five steps. The specific synthetic route is as follows: the two secondary alcohols (3.1) in uridine are protected to acetonide (acetonide) using 2,2-dimethoxypropane in acetone under the action of sulfuric acid catalysis. Dynamic crystallization, quenching with triethylamine, and subsequent addition of n-heptane gave the acetonide product in 91% yield on a metric ton scale. The 5′-isobutyryl group is then introduced (using isobutyric anhydride, triethylamine, 4-dimethylaminopyridine, and ethyl acetate) to generate the ester 3.2, which is processed in the form of an acetonitrile solution. The solvent switch from ethyl acetate to acetonitrile eliminates the risk of product precipitation during storage and slightly increases the reaction rate of the subsequent steps. The C4 carbonyl of the uracil ring on the intermediate 3.2 is converted to the corresponding oxime (3.4) in two steps. The amide carbonyl is first activated using a triazole phosphate reagent generated in situ from 1,2,4-triazole, diisopropylethylamine, and phosphorus trichloride in acetonitrile. Due to the nucleophilicity of 1,2,4-triazole at both N1/N2 (desired position) and N4 (undesired position), a mixture of triazole isomers was generated initially in the reaction, but in the presence of excess triazole, it was converted to the thermodynamically more stable desired isomer (ratio >99:1) within a few hours. Crystallization using water as an antisolvent provided the triazole intermediate 3.3 in 92% yield over a two-step process. Subsequent workup included treatment with water-soluble hydroxylamine and crystallization directly after addition of water to afford the final oxime 3.4 in 91% yield. Finally, removal of the acetonide protecting group under acidic conditions (HCl, water, and MeCN) afforded Molnupiravir (3). This deprotection step required extensive optimization to minimize hydrolysis of the acid-sensitive ester and oxime functionalities. The final active pharmaceutical ingredient was crystallized by slow addition of ethyl acetate to obtain a high purity product in 76% overall yield (57% from uracil in 5 steps).
Regulatory Status
Molnupiravir (as EIDD-2801) was developed at Drug Innovation Ventures at Emory (DRIVE), a not-forprofit biotechnology company owned by Emory University, Atlanta. It was then licensed by Ridgeback Biotherapeutics, and is now developed further in cooperation with Merck & Co. (in Europe: Merck Sharp & Dohme/ MSD).
Molnupiravir is not approved by the European Medicines Agency (EMA) or the American Food and Drug Administration (FDA).
Einzelnachweise
[1] Toots M, Yoon J-J, Cox RM et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Science Translational Medicine 2019;11(515). Epub 23 Oct 2019.
[2] Toots M, Yoon J-J, Hart M et al. . Quantitative Efficacy Paradigms of the Influenza Clinical Drug Candidate EIDD-2801 in the Ferret Model. Transl Res 2020. Epub 2019 Dec 25.
[3] Halford B. An emerging antiviral takes aim at COVID-19. American Chemical Society; 2020 [14.12.2020]; Available from:
https://cen.acs.org/pharmaceuticals/drug-development/emergingantiviral-takes-aim-COVID-19/98/web/2020/05. [4] Ridgeback Biotherapeutics. OVERVIEW OF EIDD-2801 (MK-4482). [Presentation]. provided via email 2020.
Molnupiravir Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

