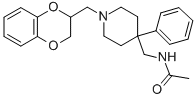
Acoxatrine
- CAS No.
- 748-44-7
- Chemical Name:
- Acoxatrine
- Synonyms
- Acoxatrine;N-[[1-(2,3-dihydro-1,4-benzodioxin-2-ylmethyl)-4-phenyl-4-piperidyl]methyl]acetamide;N-[[1-(2,3-dihydro-1,4-benzodioxin-2-ylmethyl)-4-phenylpiperidin-4-yl]methyl]acetamide;N-[[1-(2,3-dihydro-1,4-benzodioxin-2-ylmethyl)-4-phenyl-piperidin-4-yl]methyl]ethanamide
- CBNumber:
- CB01117536
- Molecular Formula:
- C23H28N2O3
- Molecular Weight:
- 380.485
- MDL Number:
- MOL File:
- 748-44-7.mol
| FDA UNII | 7IGS0KX75Q |
|---|
Acoxatrine price
Acoxatrine Chemical Properties,Uses,Production
Originator
Acoxatrine ,ZYF Pharm Chemical
Manufacturing Process
To a solution of 11.5 parts lithium aluminum hydride in 100 parts
tetrahydrofurane is added dropwise a solution of 94 parts DL-1-cyano-4-
phenyl-piperidine in 240 parts tetrahydrofurane, at a temperature of about
45°C. After the addition is complete, the reaction mixture is stirred first at the
same temperature for 3 h and 30 min and then refluxed for 1 h. The whole is
decomposed by successive addition of 12 parts water, 9 parts sodium hydroxide 20% and 50 parts water. The mixture is filtered from inorganic
matter. The filter-cake is washed with tetrahydrofurane and the combined
filtrates are evaporated. The oily residue is dissolved in 240 parts 2-propanol
and to this solution are added about 60 parts concentrated hydrochloric acid.
After keeping at room temperature, the precipitated salt is filtered off, washed
with 2-propanol and dried, yielding DL-4-(amino-methyl)-1-[1,4-
benzodioxanyl)methyl]-4-phenylpiperidine dihydrochloride; melting point
272°-278°C as a white amorphous powder.
From 4.1 parts DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4-
phenylpiperidine dihydrochloride, the free base is liberated in the usual
manner and extracted with chloroform. The organic layer is separated, dried
and evaporated. The DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4-
phenylpiperidine obtained is dissolved in 128 parts anhydrous chloroform. This
solution is cooled to 5°C and there is added dropwise a solution of 1.6 parts
acetylchloride in 7 parts anhydrous chloroform (exothermic reaction). The
reaction mixture is stirred over night at room temperature and then alkalized
with about 25 parts sodium hydroxide 20% at a temperature of 20°C. The
aqueous layer is separated and extracted twice with chloroform. The combined
organic layers are washed with water, dried over magnesium sulfate, filtered
and evaporated. The oily residue is dissolved in a mixture of 40 parts acetone
and 20 parts diisopropyl ether and evaporated again. The solid residue is
triturated in diisopropylether, yielding DL-4-(N-acetylaminomethyl)-1-[2-(1,4-
benzodioxanyl)-methyl]-4-phenylpiperidine; melting point 140°-141.1°C, as a
white microcrystalline powder.
Therapeutic Function
Vasodilator, Antihypertensive
Acoxatrine Preparation Products And Raw materials
Acoxatrine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Leancare Ltd. | -- | enquiry@leancare.co.uk | United Kingdom | 6460 | 42 |
| Lanospharma Laboratories Co.,Ltd | -- | sales@lanospharma.com | China | 6343 | 56 |
| 2A PharmaChem USA | -- | sales@2apharmachem.com | United States | 6148 | 39 |
| 3B Scientific Corporation | -- | sales@3bsc.com | United States | 6744 | 47 |
| Supplier | Advantage |
|---|---|
| Leancare Ltd. | 42 |
| Lanospharma Laboratories Co.,Ltd | 56 |
| 2A PharmaChem USA | 39 |
| 3B Scientific Corporation | 47 |