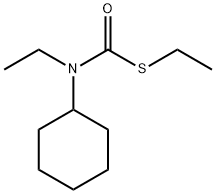
CYCLOATE
- CAS No.
- 1134-23-2
- Chemical Name:
- CYCLOATE
- Synonyms
- SABET;EUREX;Ronit;etsan;R-2063;RO-NEET;CYCLOATE;Cycolate;ro-neete;AGRO-NET
- CBNumber:
- CB0134167
- Molecular Formula:
- C11H21NOS
- Molecular Weight:
- 215.36
- MDL Number:
- MFCD00055341
- MOL File:
- 1134-23-2.mol
- MSDS File:
- SDS
| Melting point | 11.5°C |
|---|---|
| Boiling point | 145 °C(Press: 10 Torr) |
| Density | 1.0156 |
| vapor pressure | 0.002Pa at 25℃ |
| refractive index | 1.5500 (estimate) |
| Flash point | >100 °C |
| storage temp. | 0-6°C |
| form | Liquid |
| pka | -1.30±0.20(Predicted) |
| Water Solubility | 85mg/L(22 ºC) |
| BRN | 2937178 |
| LogP | 3.88 at 20℃ |
| CAS DataBase Reference | 1134-23-2 |
| EWG's Food Scores | 1 |
| FDA UNII | IMZ37NA07H |
| Proposition 65 List | Cycloate |
| Pesticides Freedom of Information Act (FOIA) | Ro-Neet |
| EPA Substance Registry System | Cycloate (1134-23-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H331-H411 |
| Precautionary statements | P261-P271-P273-P304+P340+P311-P391-P403+P233 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-51/53 |
| Safety Statements | 61 |
| RIDADR | UN3082 9/PG 3 |
| WGK Germany | 2 |
| RTECS | GU7200000 |
| Toxicity | LC50 (96-hour) for rainbow trout 4.5 mg/L (Hartley and Kidd, 1987), for mosquito fish 10 ppm (Humburg et al., 1989); acute oral LD50 of technical cycloate for male and female rats 2,000–3,190 and 3,160–4,100 mg/kg, respectively (Hartley and Kidd, 1987), 1,678 mg/kg (RTECS, 1985) |
CYCLOATE price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 45408 | Cycloate PESTANAL | 1134-23-2 | 250mg | $63.1 | 2024-03-01 | Buy |
| TRC | C989320 | Cycloate | 1134-23-2 | 500mg | $130 | 2021-12-16 | Buy |
| TRC | C989320 | Cycloate | 1134-23-2 | 5g | $1050 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PST0000348 | CYCLOATE 98.00% | 1134-23-2 | 10G | $1536.15 | 2021-12-16 | Buy |
| AHH | MT-49579 | Cycloate 98% | 1134-23-2 | 10g | $360 | 2021-12-16 | Buy |
CYCLOATE Chemical Properties,Uses,Production
Chemical Properties
Cycolate is an oily, clear, or amber to yellow liquid. Aromatic odor;
Uses
Herbicide used to control several broad-leaved weeds and many annual grasses in sugar beets, table beets and spinach
Definition
ChEBI: Cycloate is a primary aliphatic amine.
General Description
Colorless liquid with an aromatic odor. Used as a selective systemic herbicide.
Air & Water Reactions
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids or alkalis.
Reactivity Profile
CYCLOATE is a thiocarbamate ester. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.
Flammability and Explosibility
Non flammable
Agricultural Uses
Herbicide: Used to control broadleaf weeds, annual and perennial grasses and nutgrass in spinach, beets, and sugar beets. Not approved for use in EU countries. Actively registered in the U.S.
Trade name
ETSAN®; EUREX®; R-2063®; RO- NEET®; RO-NEET®-6E; RO-NEET® 10G; RONIT®; SABET®
Potential Exposure
Cycolate is a thiocarbamate herbicide used to control broad leaf weeds, annual and perennial grasses and nutgrass in spinach, beets, and sugar beets.
Environmental Fate
Soil. The reported half-life in soil is approximately 4–8 weeks (Hartley and Kidd,
1987)
Groundwater. According to the U.S. EPA (1986) cycloate has a high potential to leach
to groundwater
Plant. Cycloate is rapidly metabolized in sugarbeets to carbon dioxide, ethylcyclohexylamine, sugars, amino acids and other natural constituents (Humburg et al., 1989)
Chemical/Physical. In the gas phase, cycloate reacts with hydroxyl and NO3 radicals
but not with ozone. With hydroxy radicals, cleavage of the cyclohexyl ring was suggested
leading to the formation of a compound tentatively identified as C2H5(CHO)NC(O)SC2H5.
The calculated photolysis lifetimes of cycloate in the troposphere with hydroxyl and NO3
radicals are 5.2 hours and 1.4 days, respectively (Kwok et al., 1992).
Shipping
UN3082 Environmentally Hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required
Incompatibilities
Cycolate reacts violently with powerful oxidizers such as calcium hypochlorite. Thiocarbamate esters are combustible. Poisonous gases are generated by the thermal decomposition of thiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitro- gen, hydrogen sulfide, ammonia, and methylamine. Thio and dithiocarbamates slowly decompose in aqueous solu- tion to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thio- carbamates with aldehydes, nitrides, and hydrides. Thiocarbamates are incompatible with acids, peroxides, and acid halides.
Waste Disposal
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guid- ance on acceptable disposal practices. Incineration with effluent gas scrubbing is recommended. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
CYCLOATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | cherry@crovellbio.com | China | 18444 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| BEST-REAGENT | 400-1166-196 18981987031 | cdhxsj@163.com | China | 11726 | 57 |
| Chengdu Ai Keda Chemical Technology Co., Ltd. | 4008-755-333 18080918076 | 800078821@qq.com | China | 9725 | 55 |